
Expired activity
Please go to the PowerPak
homepage and select a course.

Adjusting Background Therapy when Initiating GLP-1 Receptor Agonists: What Pharmacists Need to Know (Module #2)
Learning Objective:
- Explain strategies for managing background therapies when starting a GLP-1 RA in a patient with type 2 diabetes to improve glycemia and/or mitigate cardiovascular and kidney risk.
The role of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in the management of type 2 diabetes mellitus (T2D) continues to evolve. Based on evidence from completed cardiovascular and kidney outcome trials, organizations like the American Diabetes Association (ADA), the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE), the American College of Cardiology (ACC), and Kidney Disease: Improving Global Outcomes (KDIGO) note the glycemic, weight, and organ protective benefits of GLP-1 RAs.1-4 Figure 1 provides a summary of the ADA algorithm for intensification of glucose-lowering medications in T2D.1 The ADA currently provides the following guidance and recommendations for GLP-1 RAs use in patients with T2D:1
- A GLP-1 RA or a sodium-glucose cotransporter2 (SGLT2) inhibitor with proven cardiovascular benefit is recommended in patients with T2D with established atherosclerotic cardiovascular disease (ASCVD) or indicators of high ASCVD risk.
- A GLP-1 RA with proven cardiovascular benefit is recommended in patients with T2D who have albuminuric diabetic kidney disease (DKD) if a SGLT2 inhibitor is contraindicated or not tolerated. A GLP-1 RA with proven cardiovascular benefit is also recommended as an option in patients with T2D and chronic kidney disease (CKD) to mitigate the risk of cardiovascular events.
- GLP-1 RAs are recommended to improve glycemia when there is a compelling need to minimize hypoglycemia and/or minimize weight gain or promote weight loss.
- GLP-1 RAs are recommended as the first injectable agent (over insulin) in most patients with T2D when the glucose-lowering efficacy of an injectable agent is desired.
| Figure 1. American Diabetes Association (ADA) Algorithm for Intensification of Glucose-Lowering Medications in Type 2 Diabetes. (Adapted from Reference #1) |
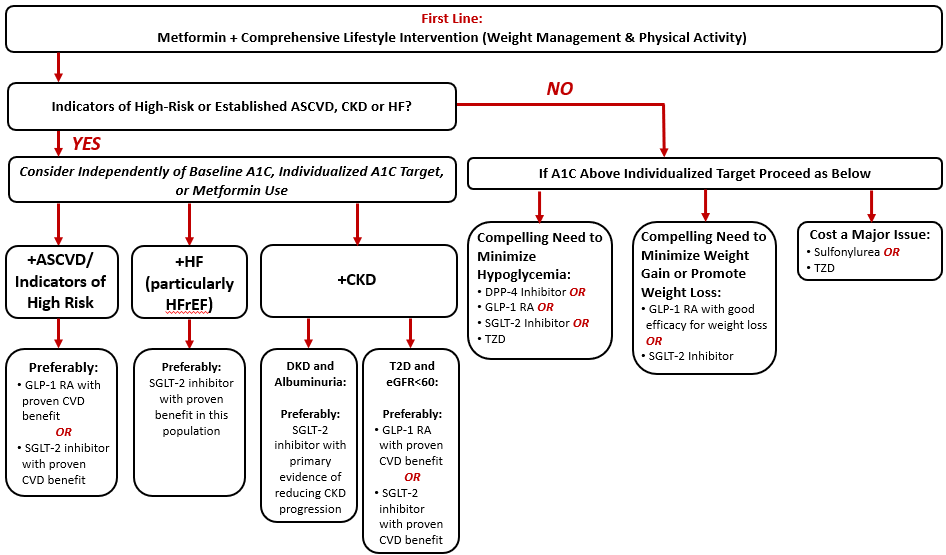 |
| Abbreviations: A1C, glycated hemoglobin A1c; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; SGLT-2, sodium-glucose cotransporter 2; T2D, type 2 diabetes mellitus; TZD, thiazolidinedione. |
While current guidelines are clear regarding the benefits of GLP-1 RAs in patients with T2D, there is less clarity about how to adjust background therapies when initiating a GLP-1 RA. For example, while GLP-1 RAs carry a low intrinsic risk of hypoglycemia, the addition of a GLP-1 RA to background sulfonylurea or insulin therapy can increase the risk for treatment-emergent hypoglycemia.4 Accordingly, the manufacturer prescribing information for all currently available GLP-1 RAs includes a warning for increased risk of hypoglycemia when used in combination with an insulin secretagogue or insulin5-11 and state that reducing the dose of the insulin secretagogue or insulin may be necessary to prevent hypoglycemia. While a general warning stating that reducing the dose of background insulin secretagogue or insulin may be necessary to prevent hypoglycemia is listed in each product insert, specific guidance on how to dose adjust background therapies is not detailed.
The following information can be used to inform adjustment of background therapies in a patient starting a GLP-1 RA, with the approach individualized based on the specific needs of each patient.
GLP-1 RA Addition to Background Insulin
The combination of a GLP-1 RA with basal insulin is commonly used in patients with T2D and is a recommended combination in major treatment guidelines to meet individualized treatment goals in appropriate patients.1,2 As previously noted, when adding a GLP-1 RA to established background basal insulin therapy, adjustment of the background insulin is often necessary to avoid hypoglycemia. In clinical trials where a GLP-1 RA was added to background basal insulin, a general 20% basal insulin reduction in all patients or a 20% reduction in patients with an A1C < 8.0% were often implemented to avoid hypoglycemia.12-14 Based on this approach, it has been suggested in the literature that for patients on a stable basal insulin regimen who have an A1C ≤ 8.0%, the basal insulin should be reduced empirically by approximately 20% when starting a GLP-1 RA.15 For those with an A1C > 8.0%, the GLP-1 RA can be reasonably initiated without a reduction in the basal insulin dose for many patients. In those with a history of severe hypoglycemia and/or hypoglycemia unawareness, however, it may be prudent to reduce the basal insulin dose to err on the side of safety regardless of their current A1C. Following GLP-1 RA initiation at the lowest dose with subsequent titration, the basal insulin dose can then be titrated based on clinical response and as informed by blood glucose monitoring (BGM) data.
For patients on mealtime insulin, the addition of a long-acting GLP-1 RA can result in a decreased need for prandial insulin. In fact, in a recently published study in patients with T2D receiving basal/bolus insulin, the addition of a once-weekly GLP-1 RA resulted in more than half of participants no longer requiring mealtime insulin after starting GLP-1 RA therapy.16 Therefore, adjustment of background mealtime insulin is important to prevent hypoglycemia when starting a GLP-1 RA. In the trial described above, mealtime insulin was reduced by 50% when starting the GLP-1 RA followed by complete discontinuation after 4 weeks. The mealtime insulin was reintroduced at week 8 of the trial in those requiring additional postprandial glucose control.16 Based on these data, it is reasonable to decrease background prandial insulin doses by 50%, or even hold the prandial insulin altogether in patients with “good” glucose control or a history of hypoglycemia when starting a GLP-1 RA. Eventual discontinuation or retitration of the prandial insulin can then be informed by patient response to therapy and BGM data.
GLP-1 RA Addition to Background Sulfonylurea Therapy
Similar to the discussion above about background insulin, it is prudent in many cases to hold or reduce the dose of background sulfonylurea therapy (an initial 50% dose reduction is reasonable in many patients) when starting a GLP-1 RA.17,18 In the authors’ experience, it is often possible to eliminate the sulfonylurea altogether, which can be advantageous in terms of avoiding hypoglycemia, minimizing the complexity of the diabetes regimen, and augmenting the weight loss benefits of the GLP-1 RA. Whether it is decided to initially decrease the dose or hold the background sulfonylurea altogether, continued use and titration of the sulfonylurea should be informed by patient response and BGM data.
GLP-1 RA Addition to Background DPP-4 Inhibitor
GLP-1 RAs and DPP-4 inhibitors both work to augment the impaired incretin response present in people with T2D. While using a medication from each class in combination does not pose a safety concern for patients, there is no evidence supporting additive efficacy with this combination. Thus, combined use is not recommended.1,2 If initiating a GLP-1 RA in a patient with T2D for glucose-lowering effect and/or for organ protection in a patient on background DPP-4 inhibitor therapy, it is recommended that the DPP-4 inhibitor be discontinued.1
Case Scenario
RY is a 64-year-old man with T2D of 14-years’ duration. His diabetes regimen currently consists of insulin glargine (U-100) 40 U subcutaneously once daily in the evening, metformin ER 1000 mg twice daily, and sitagliptin 100 mg daily. RY’s current A1C is 7.5%. His past medical history is positive for obesity and hypertension.
RY’s primary care provider (PCP) would like to start him on semaglutide 0.25 mg once weekly to improve his A1C and to assist with weight loss. RY’s PCP asked pharmacy for recommendations on adjusting RY’s background therapy when starting the GLP-1 RA.
The following recommendations were made:
- Discontinue sitagliptin 100 mg daily.
- Because RY’s A1C is currently 7.5% (ie, < 8.0%), reduce his insulin glargine from 40 U once daily to 32 U once daily (initial empiric dose reduction of 20%).
- Follow up with RY within the next week to discuss his response to the GLP-1 RA (glycemic response and tolerability); consider further down-titration of the insulin glargine with any noted hypoglycemia.
- Review the signs and symptoms of hypoglycemia with RY and appropriate methods for treatment hypoglycemic events should they occur (eg, Rule of 15).
|
In conclusion, GLP-1 RAs provide an important therapeutic option for patients with T2D to improve glucose control, mitigate cardiovascular and kidney risk, and facilitate weight loss. When starting a GLP-1 RA it is important to consider adjustment of background therapies to avoid treatment emergent hypoglycemia and/or unnecessary therapeutic duplication. Pharmacists can play a critical role as members of the diabetes management team by recommending appropriate background therapy adjustments and facilitating medication titration as appropriate per patient response.
References
- American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care.2021;44(suppl 1):S1-S232.
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract. 2020;26(1):107-139.
- Das SR, Everett B, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1-S115.
- Byetta (exenatide) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
- Adlyxin (lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis US LLC; 2019.
- Victoza (liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2020.
- Bydureon (exenatide extended-release) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020.
- Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Co; 2021.
- Ozempic (semaglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk, Inc; 2021.
- Rybelsus (semaglutide) tablets [product information]. Plainsboro, NJ: Novo Nordisk, Inc; 2021.
- Mathieu c, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16(7):636-644.
- Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017;19(7):1024-1031.
- Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103-112.
- Carris NW, Talor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74:2141-2152.
- Rosenstock J, Nino A, Soffer J, et al. Impact of a weekly glucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabetes Care. 2020;43:2509-2518.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083-1091.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628-2635.
Back to Top