
Expired activity
Please go to the PowerPak
homepage and select a course.
Finding the Recipe for Success in Diabetes: Achieving Targets with Oral Combination Therapy
Introduction
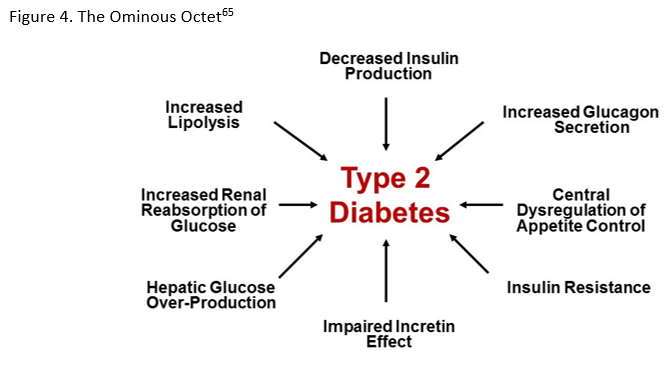
Among the estimated 30 million individuals with diabetes in the United States (U.S.), 90-95% have type 2 diabetes mellitus (T2DM).1 Despite the existence of evidence-based guidelines and new treatment modalities, many patients do not achieve individualized glycemic targets and are at risk for diabetes-related complications. Clinical inertia frequently delays treatment intensification.2-4 It is a complicated phenomenon that has been attributed to physicians' underestimation of the extent of patients not at goal, physicians' reluctance to adopt newer individualized targets, patient non-adherence to pharmacologic and non-pharmacologic treatment, and concerns related to drug safety and efficacy.5 Achievement of individualized glycated hemoglobin (A1C) targets for patients with diabetes have declined from 69.8% (95% CI 66.5-73.0%) to 63.8% (95% CI 60.1-67.5%) based on data from the National Health and Nutrition Examination Survey.6 Also, the percentage of patients with A1C >9.0% has increased from 12.6% (95% CI 10.5-14.8%) to 15.5% (95% CI 12.9-18.2%).6
For many people with T2DM, combination therapy is required to reach individualized glycemic goals. In fact, patients presenting with very high A1C or certain comorbidities may benefit from initial combination therapy. 7
Pharmacologic management guidelines now offer specific direction when choosing glucose-lowering therapy based on the presence of atherosclerotic cardiovascular disease (ASCVD) or indicators of high ASCVD risk, heart failure (HF), or chronic kidney disease (CKD).8 For other patients, treatment can be based on the need to minimize hypoglycemia, promote weight loss/avoid weight gain, or to limit drug costs.8 Among oral glucose-lowering medications, DPP-4 inhibitors and SGLT-2 inhibitors have important roles in guideline recommendations for patient management based on their individual and combined characteristics.
It is important for pharmacists to understand the place in therapy for these agents and the data that supports their combined use. Several fixed-dose combinations (FDCs) of DPP-4 inhibitor/SGLT2 inhibitors are currently available to patients. Multidisciplinary, team-based care is essential in diabetes management. Pharmacists have played an important role in diabetes care for many years and with the continually growing population of patients with diabetes, coupled with the declining availability of physicians, pharmacists will be even more important in meeting clinical needs. Because patients with T2DM often present with barriers to medication adherence, pharmacists should be familiar with FDCs that can improve medication adherence in some patients when used in conjunction with treatment and educational interventions that may help achieve individualized glycemic targets sooner.7
RECOMMENDATIONS FOR COMBINATION THERAPY IN THE MANAGEMENT OF T2DM
In recent years, treatment algorithms providing recommendations for selection of glucose-lowering therapies in patients with T2DM have undergone many alterations based on recent clinical trial evidence, and several new therapeutic options have reached the market. After initial treatment failure, typically with metformin, the American Diabetes Association (ADA) algorithm now gives preference to specific classes of agents based on a patient's comorbidities and characteristics.
Review of Guideline Recommendations
The ADA's Standards of Medical Care in Diabetes have traditionally advocated for a stepwise approach of adding glucose-lowering agents when initial treatment is unsuccessful. Benefits of this approach include the ability to assess the positive and negative effects of new drugs and reduce patient expense.8 Therapy should be reassessed every 3 to 6 months and modified as needed. The 2020 ADA Standards of Care continue to list metformin and lifestyle interventions as the preferred first-line therapy, in the absence of a contraindication, and promote regular treatment modification to avoid therapeutic inertia.8 Following first-line therapy, the ADA now recommends the addition of glucose-lowering therapies to both lower glycemia (A1C) and reduce cardiovascular and renal risk independent of A1C.8 First, in patients with ASCVD or indicators of high ASCVD risk, a GLP-1 receptor agonists (RA) or an SGLT-2 inhibitor (if eGFR is adequate) with demonstrated CVD benefit is recommended as an addition (see Table 1). This is recommended for consideration independent of A1C and the need for additional glucose lowering. For patients in whom HF or CKD predominates, the addition of an SGLT2 inhibitor with evidence of reducing HF and/or CKD progression is recommended; if an SGLT2 inhibitor cannot be taken due to a contraindication a GLP-1 RA with proven CVD benefit is recommended for addition independent of the need for additional glucose lowering. 8 Second, in patients without these factors who need additional glucose lowering to meet individualized glycemic goals, risk of hypoglycemia, effect on weight, cost, and patient preferences should be considered when selecting an add-on agent (see Table 2.
While the ADA continues to largely advocate for a stepwise approach, evidence is mounting that this approach may not be optimal in all patients. 9 Indeed a growing body of evidence supports the initial use of combination therapy in some patients to improve hyperglycemia, optimize long-term outcomes, and delay disease progression.10-12 Now the ADA recommendations state "early combination therapy can be considered in some patients at treatment initiation to extend the time to treatment failure." 8 Agent selection should follow the same guidance described for sequential drug addition. One goal of initial combination therapy is to achieve glycemic targets rapidly. The benefits of early goal achievement, which will be discussed later, and risks of extended time in hyperglycemia support the use of early combination therapy.
The presence of indicators of high-risk or established ASCVD, HF, or CKD should be considered very early in the selection of glucose-lowering therapies.13 Because these considerations are independent of baseline A1C or A1C target, it may be appropriate to begin treatment with an agent with proven benefit in addition to metformin and lifestyle modification. In other words, agents with proven benefit can be added even when patients are at target A1C and/or it is prior to the typical 3-month assessment period.8
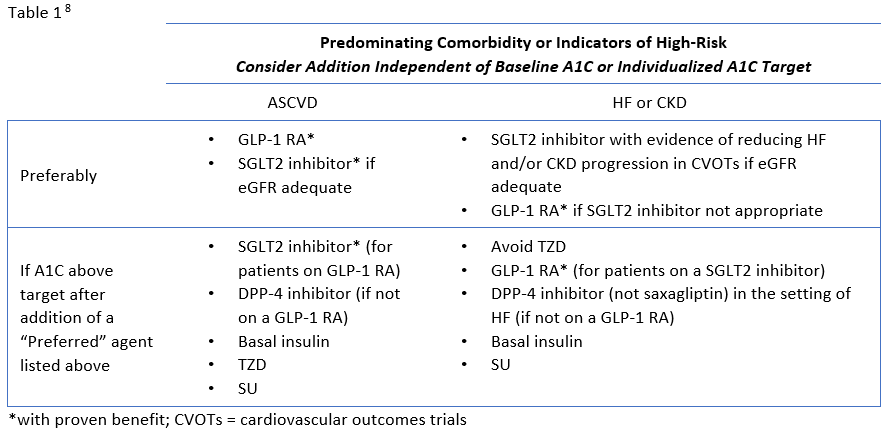
The newer classes of oral agents have predominant roles in the context of glucose lowering as well as comprehensive cardiovascular and renal risk reduction. Notably, SGLT2 inhibitors that have demonstrated benefit from outcomes trials are preferred in patients with HF or CKD, and are also recommended as an option in patients with established ASCVD or indicators of high risk.8 DPP-4 inhibitors are also options for add-on therapy for additional glucose-lowering, but are not recommended to be used in combination with a GLP-1 RA (Table 1).8 Both SGLT2 inhibitors and DPP-4 inhibitors are preferred options when there is a need to minimize hypoglycemia. Weight loss associated with SGLT2 inhibitors and the neutral weight effect of DPP-4 inhibitors make both appropriate for patients who need to lose weight or minimize weight gain.8
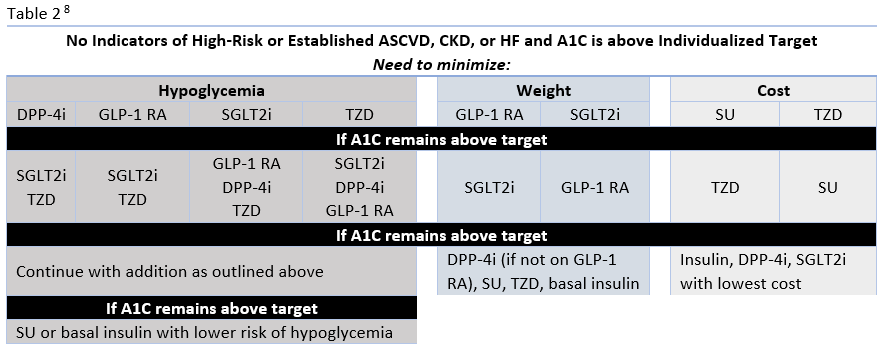
Factors to Consider for Treatment Selection
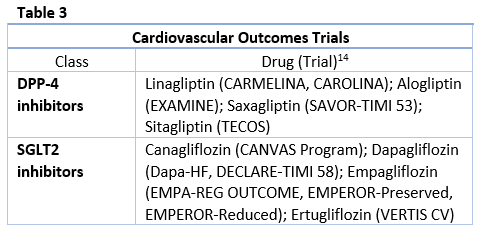
SGLT2 inhibitors and DPP-4 inhibitors are among the newest oral antidiabetic drugs indicated for T2DM in the U.S. These drugs both have unique, non-insulin dependent mechanisms of action along with favorable adverse effect profiles in terms of weight gain and hypoglycemia, making them attractive additions to the diabetes pharmacotherapy armamentarium. Data on cardiovascular outcomes (see Table 3) and renal effects are currently available, with more information to come. These newer classes also have unique risks that should be taken into consideration when selecting therapy and educating patients. Data on these classes are provided below.
SGLT2 Inhibitors
SGLT2 inhibitors reduce blood glucose by reducing reabsorption of filtered glucose and lowering the renal threshold for glucose excretion, allowing more glucose to be excreted in the urine.15 Because of their insulin-independent mechanism of action, SGLT2 inhibitors are appropriate for use in a wide spectrum of patients, regardless of baseline A1C levels or duration of diabetes.15 SGLT2 inhibitors that are currently approved in the U.S. include canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin.16
There are many beneficial effects of treatment with SGLT2 inhibitors. Meta-analysis of canagliflozin, dapagliflozin, and empagliflozin showed reductions in A1C that range from 0.6% to 0.9%.17 In a 2019 analysis of ertugliflozin 5 mg and 15 mg, reductions in A1C were similar (0.8% and 0.9%, respectively).18 As previously mentioned, low hypoglycemic risk is an important benefit of SGLT2 inhibitors, as is weight loss and reductions in blood pressure.16 Calories lost with the extra glucose that is excreted in the urine contributes to the initial weight loss seen with SGLT2 inhibitors and sustained weight reduction is largely due to a reduction in fat mass.16 The weight reductions, which are typically 2 to 3 kg, have been shown to be statistically significant versus placebo for all approved agents.18-21
Clinically significant reductions in blood pressure achieved with SGLT2 inhibition may be due to osmotic diuresis, weight changes, improvements in vascular stiffness, reduced sympathetic nervous system activity, and lower serum uric acid concentrations.16,22 Small dose-related increases in low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) can occur, and also reductions in tryglycerices.16
CVOTs have demonstrated ASCVD benefits for canagliflozin and empagliflozin and reduction in hospitalization for HF for empagliflozin, canagliflozin, and dapagliflozin.23-27 In the EMPA-REG Outcome Trial, empagliflozin treatment demonstrated a relative risk reduction in major adverse cardiovascular events (MACE) (14%), cardiovascular mortality (38%) and all-cause mortality (32%).23 The CANVAS trial with canagliflozin showed reduced cardiovascular events compared with placebo. 27 In the DECLARE-TIMI 58 trial, dapagliflozin resulted in a lower rate in the composite outcome of cardiovascular death or hospitalization for HF in patients with T2DM who had or were at risk for ASCVD. It did not, however, detect a difference in the MACE rate between dapagliflozin and placebo.26 Results from the VERTIS-CV trial will provide data on the cardiovascular and renal safety and efficacy of ertugliflozin.
Information on renal protective effects are also emerging for these drugs. The existing CVOTs revealed several important pieces of information:
- DECLARE-TIMI 58 trial - lower rates of progression of renal disease with dapagliflozin26
- EMPA-REG OUTCOME - higher eGFR, reduced development of acute renal failure, and reduced albuminuria with empagliflozin28
- CANVAS - reduction in progression of albuminuria and death from renal causes with canagliflozin28
While the mechanisms responsible for the positive renal outcomes seen to date with SGLT2 inhibitors remain unknown, they are known to cause vasoconstriction of the afferent arterioles. This decreases hyper-filtration in the glomerulus, thus leading to a decrease in the rate of progression of proteinuria.29 While it is likely that several other mechanisms may contribute to the renal sparing of effects of SGLT2 inhibition, mitigation of glomerular hyper-filtration is theorized to contribute at least in part. The CREDENCE study was the first renal outcome trial completed with an SGLT2 inhibitor. In this trial, canagliflozin demonstrated lower risk of the primary outcome (a composite of progression to need for dialysis, transplantation, or a sustained estimated GFR of <15 ml/min/1.73m2) and lower relative risk of progressing to end-stage kidney disease.30 Based on these benefits, canagliflozin is now approved to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hospitalization for HF in adults with T2DM and diabetic nephropathy with albuminuria.31 Clinical trials in CKD populations are currently ongoing for dapagliflozin (Dapa-CKD) and empagliflozin (EMPA-KIDNEY). 32
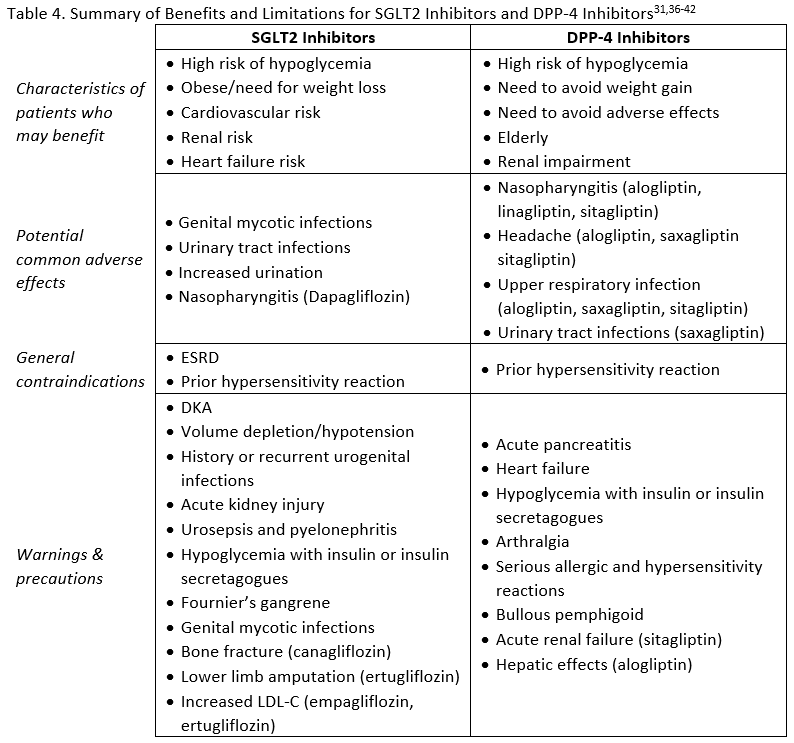
Adverse effects, contraindications, and warnings for SGLT2 inhibitors are listed in Table 4. Although the general safety profile of SGLT2 inhibitors appears to compare favorably with that of other agents used to treat T2DM, a few notable risks exist that are unique to this class. Genital mycotic infections and urinary tract infections related to glycosuria are two adverse events that need to be considered in patients receiving treatment with an SGLT2 inhibitor.15 Counseling patients about genital hygiene is likely to minimize the risk of infection.33 Fournier's gangrene (FG) is a rare but serious risk in patients taking SGLT2 inhibitors. Also called necrotizing fasciitis of the perineum, it is a rapidly progressive necrotizing infection of the external genitalia, perineum, and perianal region requiring broad-spectrum antibiotics and immediate surgical intervention.34 Between March 2013 and January 2019 the FDA identified 55 cases in patient taking SGLT2 inhibitors.34 The critical nature of this condition requires awareness of the risk by clinicians and provision of education to patients about FG. As noted previously, osmotic diuresis due to SGLT2 inhibitor use can be beneficial in reducing blood pressure, but in patients with low systolic blood pressure, renal impairment, diuretic use, or the elderly it may contribute to hypotention.15 Diabetic ketoacidosis (DKA) has been reported with SGLT2 inhibitors. Many of the cases were in insulin deficient patients (e.g., type 1 diabetes, excessive insulin dose reductions in insulin-treated patients) but DKA can also occur in patients with T2DM experiencing metabolically stressful events.15 Additional risks include low carbohydrate diet, malnourishment, alcohol abuse, surgery, and infection.33 Some of the cases with SGLT2 inhibitors have been euglycemic DKA. This is DKA without the typically associated hyperglycemia.35 This is particularly challenging because many patients do not recognize euglycemic DKA and clinicians my misdiagnose/mistreat DKA with normal glucose levels.35
There are possible effects of SGLT2 inhibitors on the renal tubular transportation of bone minerals.33 An increased risk of bone fracture has been observed in patients using canagliflozin. The CANVAS program showed a 26% relative increase in risk of bone fracture with canagliflozin compared to placebo.27 Also, a nearly two-fold increase in lower-leg amputation was seen in the CANVAS program. 27 Due to these safety concerns, the FDA issued a safety for fractures and a black box warning in the canagliflozin product labeling regarding the risk of lower limb amputations.31
It should be noted, however, that in the CREDENCE study significant differences were not seen in rates of fracture or amputation between participants receiving canagliflozin or placebo.30 The reason for these disparate findings from these two large studies is currently unknown. The glucose lowering effects of SGLT2 inhibitors is dependent on renal function. Therefore, there are renal dosing recommendations within the labeling for each product when used for their glucose lowering effects. Please refer to the prescribing information for each product for up-to-date renal dosing recommendations.
DPP-4 Inhibitors
DPP-4 inhibitors work by delaying the breakdown of incretin hormones, GLP-1 and GIP, by the enzyme DPP-4 which normally occurs within minutes after food ingestion. Through prolongation of the effects of endogenously secreted incretin hormones, DPP-4 inhibitors increase post-prandial insulin secretion, reduce glucagon secretion, reduce both fasting and post-prandial serum glucose, decrease A1C, and improve β cell function.43 DPP-4 inhibitors may also increase β cell mass and protect against β cell apoptosis.44 Alogliptin, linagliptin, saxagliptin, and sitagliptin are the agents that are currently available in the U.S. 15
Though DPP-4 inhibitors provide relatively modest reductions in A1C, they remain attractive options due to their favorable tolerability. In one meta-analysis, DPP-4 inhibitors were found to decrease A1C by approximately 0.77%.15 With this decrease, there were positive qualities such as low risk of hypoglycemia and weight neutrality.15 DPP-4 inhibitors can be used in renal impairment without an effect on efficacy or adverse effects.45 While all currently available agents can be used in all stages of kidney disease, including end-stage kidney disease, dose reductions are recommended with all agents except linagliptin which does not require renal dose adjustment. This class may also be a suitable option for elderly patients due to their relatively favorable side effect profile, including a low risk for contributing to hypoglycemia, which can be especially dangerous for older adults.45
The CVOTs for DPP-4 inhibitors have demonstrated cardiovascular safety with no increases seen in major adverse cardiovascular events.15,45 Data is conflicting, however, for HF. The SAVOR-TIMI 53 trial found increased risk of hospitalization for HF with saxagliptin compared to placebo.15 A meta-analysis that included SAVOR-TIMI 53 and data for alogliptin from the EXAMINE trial suggested a small increased risk of HF. However, other CVOTs did not report the same result.45 Warnings for HF are included in the prescribing information for this class to encourage cautious use in patients at risk.
There has been an association with pancreatitis in some, but not all, trials of DPP-4 inhibitors, and pooled data from several large trials showed a small increased risk.45 There is also a warning regarding joint pain, which typically resolves after discontinuation of therapy.45 Adverse effects, warnings, and contraindications for DPP-4 inhibitors are summarized in Table 4. These factors should be considered, along with the potential benefits of treatment, to determine which patients would be good candidates for therapy with DPP-4 inhibitors.
When facilitating treatment decisions for many people with T2DM, combination therapy is required to reach individualized glycemic goals. In fact, patients presenting with very high A1C may benefit from initial combination therapy. It is becoming more apparent that achieving glycemic control early in the course of diabetes has an important effect on long-term outcomes, and that delays in adjusting therapy to achieve glycemic control are common in diabetes. Initial combination therapy has the potential to achieve glycemic control more rapidly and maintain control for a longer time period.5 These benefits may be attributed to preservation of β cell function and being able to target different pathophysiological defects with drugs that have different and complimentary mechanisms.5
The ADA and EASD concur that patients should be considered for initial combination therapy when there is a need to improve glycemic control rapidly and A1C is more than 1.5% above goal.7,8 Shared decision making is endorsed early in treatment so that the benefits of monotherapy versus dual therapy can be weighed. Recent recommendations in favor of initial combination therapy are largely based on the results from the VERIFY trial.7,8 This study compared metformin monotherapy, early combination therapy with metformin and vildagliptin (a DPP-4 inhibitor clinically available outside of the U.S.), and vildagliptin added to metformin if an HbA1C of 7% was not achieved. Benefits found in the patient who were randomized to initial metformin and vildagliptin combination are listed here.46 These benefits were achieved without notable differences in tolerability.46
- Lower incidence of initial treatment failure (62.1% monotherapy vs. 43.6% combination)
- Consistently lower A1C over time
- Slower deterioration of glycemic control
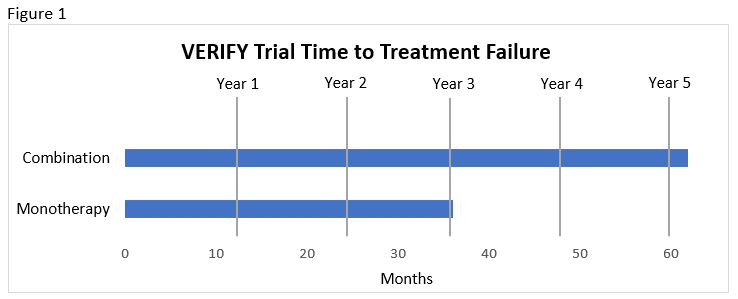
- Slower time to treatment failure compared to monotherapy and compared to sequential addition of vildagliptin to metformin (median 36.1 months with monotherapy vs. beyond the 61.9-month study duration with combination; see Figure 1).
ACHIEVING INDIVIDUALIZED GLYCEMIC GOALS: AVOIDANCE OF CLINICAL INERTIA AND MEDICATION NON-ADHERENCE
The National Health and Nutrition Examination Survey reported that only 50.9% of people with diabetes have an A1C <7.0%.6 Unfortunately, this is a persistent problem. Since 2003 only about half of patients with diabetes have achieved an A1C <7.0%, showing a need for improvement.6 Often patients do not receive prompt adjustments to their treatment regimens when glycemic parameters are not within the appropriate range. Intensification delays, often referred to as clinical inertia, can result in hyperglycemia, development of diabetic complications, higher costs, and increased risk of cardiovascular events. 9 Clinical inertia can be defined as a failure to establish appropriate targets and to escalate treatment to achieve treatment goals. 5 Delays in treatment intensification leads to extended time with hyperglycemia for many patients with T2DM.47 Elevation of A1C above 7.0% increases the risk of retinopathy with potential loss of vision; nephropathy leading to renal failure; peripheral neuropathy with risk of foot ulcers and amputations; autonomic neuropathy; sexual dysfunction; and ASCVD, peripheral arterial, and cerebrovascular disease. 8
Clinical inertia occurs in primary and specialty practice settings and impacts patients at all stages of diabetes treatment.48 The 2020 Standards of Care emphasize the importance of reducing therapeutic inertia in the management of hyperglycemia and cardiovascular disease.8
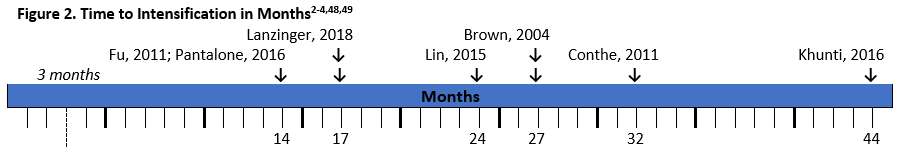
There have been several studies that demonstrate the extent of clinical inertia in diabetes management (see Figure 2). As seen in the figure, time to intensification is often longer than the recommended 3 months, and in real-world clinical practice many patients wait over a year for treatment modifications.2-4,47,48
Studies have shown that clinical inertia is greater in patients that are closer to goal and the potential for meeting glycemic goals is greater when early interventions take place. A systematic review showed that inertia increased with decreasing A1C.48 For example, a retrospective study of over 80,000 patients with T2DM found that patients with an A1C over 7% taking one oral antidiabetic drug waited 2.9 years for the addition of another glucose-lowering drug. The wait was 1.9 years when A1C was over 7.5% and 1.6 years with an A1C over 8%.50 This is significant because glycemic control as early as 3 months after diagnosis was a predictor of 5-year mortality rates.5 Also, a legacy effect may exist for achievement of early control. The UK Prospective Diabetes Study (UKPDS) showed that patients in the intensive glycemic control arm of the trial had significantly reduced rates of complications such as retinopathy, neuropathy, and nephropathy, as well as benefits on cardiovascular disease and mortality. Benefits of early intensive treatment persisted, with lower risk of diabetes-related endpoints, myocardial infarction, and death during 10-year follow-up, even when the improvements in A1C were not sustained. 5
Factors influencing clinical inertia can stem from physicians/providers or the healthcare system. Competing demands during short office visits may hinder opportunities for diabetes management.48 Physicians may overestimate the care provided or underestimate the percent of their patients who are not at glycemic goal.3,5 There may be educational or training needs due to lack of experience.3,51 System-level barriers include the cost of new medications, availability of medications, the availability of providers such as specialist nurses, and psychological support.
A review noted that among 53 articles, none included pharmacist involvement in diabetes management and that pharmacists may be able to play an important role in improving this problem. Some information is available regarding the effect of pharmacists on treatment intensification. A study including 113 patients showed that patients enrolled in pharmacist-managed diabetes clinics were able to achieve A1C, SBP, and LDL goals faster than patients receiving usual medical care.52 Another retrospective matched cohort study (N=50) showed a shorter mean time to treatment intensification in patients exposed to pharmacist care compared to the usual medical care group, which translated to greater reductions in mean A1C.53 Neither study found significant differences, however they did show the need for further investigation into the role of the pharmacist in battling clinical inertia in diabetes care.
 Clinical inertia can be linked to patient behaviors and barriers. Noncompliance with diet, socioeconomic status, acute intervening illness, terminal illness, adverse effect concerns, and treatment misunderstanding can all interfere with effective treatment.51 This "patient inertia" should be addressed by healthcare providers so that clinicians can determine the cause and take steps to alleviate the problem.3 Medication adherence is an important factor that influences treatment success. In an analysis of over 2,000 patients with T2DM, patients with the lowest adherence were significantly less likely to receive treatment intensification in a 12 month period after an elevated A1C, and those with the highest adherence had 53% greater odds of medication intensification. 54 One way that pharmacists can be involved and make a dramatic impact on diabetes care is by addressing medication adherence with patients (e.g., education, recommendations for regimen modification).3
Clinical inertia can be linked to patient behaviors and barriers. Noncompliance with diet, socioeconomic status, acute intervening illness, terminal illness, adverse effect concerns, and treatment misunderstanding can all interfere with effective treatment.51 This "patient inertia" should be addressed by healthcare providers so that clinicians can determine the cause and take steps to alleviate the problem.3 Medication adherence is an important factor that influences treatment success. In an analysis of over 2,000 patients with T2DM, patients with the lowest adherence were significantly less likely to receive treatment intensification in a 12 month period after an elevated A1C, and those with the highest adherence had 53% greater odds of medication intensification. 54 One way that pharmacists can be involved and make a dramatic impact on diabetes care is by addressing medication adherence with patients (e.g., education, recommendations for regimen modification).3
Poor patient adherence is an obstacle that makes treatment modification difficult for healthcare providers. Intensification is, however, an opportune time to assess current adherence and examine other factors that may be influencing adherence in a given patient. It has been shown that nonadherent patients are four times more likely to have poor adherence to an added drug. The risk of nonadherence to the second drug increases as the medication possession ratio (MPR) for the first drug decreases. 55
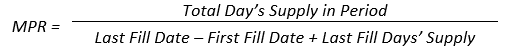
In other words, patients who do not take one medication as prescribed are not likely to take a second drug as directed. Pharmacists can intervene when new treatments are added to explore contributors to patients' nonadherence, if they exist, and adjust therapy accordingly.
Nonadherence to antihyperglycemic medications can lead to poor glycemic control. One analysis showed that for each 10% increase in the MPR, there was a mean A1C decrease of 0.24%.56 Consequences of nonadherence can include increased healthcare resource utilization (e.g., emergency department visits, inpatient admissions), higher medical costs, and higher mortality rates.57,58
A wide range of social and economic-related factors, therapy-related factors, patient-related factors, and health provider-related factors have been shown to be associated with poor adherence to diabetes medications. 57,59 These are summarized in Figure 3. The treatment regimen, if it is not convenient or patients do not understand the benefits and risks, can decrease patient motivation to take drugs as prescribed. 56,57,59 Highly complex and higher cost medications can also decrease adherence.58 Depression is a widely reported comorbidity related to nonadherence.55 Patients with depression in studies had significantly higher rates of nonadherence compared to patients who were not depressed.58 Screening for depression in patients with diabetes may help identify an important adherence barrier. 58 Distress surrounding daily life or beliefs about the consequences of diabetes can affect medication adherence as well. 56
Research shows that clinical and demographic characteristics are not strong predictors of adherence, especially with initial treatment.55 Adherence deterrents can change over time and should be assessed frequently.56 Trust in providers can also influence adherence. Pharmacists, who have frequent encounters with patients, can promote medication adherence in patients with diabetes.57 Education is needed to encourage pharmacists to initiate communication with patients about their barriers to adherence.
Approaches to Improve Medication Taking
Several approaches can be used to positively influence medication adherence rates among patients. Simplifying medication regimens by using options that are dosed once daily or using combinations that decrease pill burden may help patients improve adherence.58 FDCs, premixed insulins, and longer acting (e.g., once-weekly GLP-1 RAs) or extended-release agents (e.g., metformin ER) can help simplify treatment.56 Drug delivery devices like insulin pens or pumps can improve satisfaction and adherence.56 Studies have examined the effect of pharmacy interventions on adherence and found that refill reminders along with unit-dose packaging improved MPR and decreased physician, laboratory, and hospital services compared to controls.51,58
Above all other methods to improve medication adherence, strong communication and positive relationships with healthcare providers should be the primary goal. Providers can employ active listening, openness, provide emotional support, provide clear and thorough information, and allow adequate time for questions as strategies to promote trust with patients.56
Other communication may incorporate ways to:56
- Determine patient understanding of treatment and actual adherence
- Normalize the likelihood of poor adherence
- Address emotional distress
- Determine the influence of medication cost and affordability on medication taking behaviors
Patient motivation and commitment are important components of successful disease management and healthy behaviors.60 Not surprisingly, high patient commitment to these healthy behaviors includes medication adherence.60 Diabetes self-management education and support (DSMES) can be integrated into patient care to strengthen patient confidence, self-efficacy, commitment, and also decrease self-perceived barriers.60 DSMES can be described as " the ongoing process of facilitating the knowledge, skills, and ability necessary for diabetes self-care as well as activities that assist a person in implementing and sustaining the behaviors needed to manage his or her condition on an ongoing basis."61 Standards for DSME used for recognition by the ADA and accreditation by the American Association of Diabetes Educators (AADE) recommend that curriculum include:
- AADE7 self-care behaviors62
- Healthy eating, being active, monitoring, taking medication, problem solving, healthy coping, and reducing risks
- Diabetes pathophysiology and treatment options
- Healthy eating
- Physical activity
- Medication usage
- Monitoring and using patient-generated health data
- Preventing, detecting, and treating acute and chronic complications
- Healthy coping with psychosocial issues and concerns
- Problem solving
- Navigating the healthcare system
- Self-advocacy
This education should be customized to the patient's needs incorporating aspects such as age, cultural factors, heath literacy and numeracy, and comorbidities. Education should be ongoing to prevent diminishment of benefits and should assist patients with identifying resources that will allow them to successfully continue self-management. Monitoring of goals by educators and communicating achievement to patients is necessary to encourage their positive changes and maintenance of commitment. 61
Pharmacists can provide this type of interaction and education for patients with diabetes. Barriers related to health literacy, understanding of the disease, beliefs about disease, beliefs about treatment, and adverse effects, especially hypoglycemia, can be overcome through appropriate education and relationships with patients so that medication adherence increases and patients have the highest chance of reaching their treatment goals.
Combination Therapy with SGLT-2 inhibitors and DPP-4 Inhibitors
With proven benefit in terms of ASCVD/CKD/HF risk reduction, promotion of weight loss, and minimal risk of hypoglycemia with SGLT2 inhibitors and low hypoglycemia risk, weight neutrality, and minimal adverse effects with DPP-4 inhibitors, these medication classes can meet the individualized needs of many patients requiring combination therapy.8,16 There is also evidence supporting the safety and efficacy of these classes used together as combination therapy or as FDCs.63 SGLT2 inhibitors/DPP-4 inhibitor FDCs are described in Table 5. Examples of other FDCs with additional agents include alogliptin/metformin (Kazano), alogliptin/pioglitazone (Oseni), canagliflozin/metformin (Invokamet), dapagliflozin/metformin ER (Xigduo XR), empagliflozin/metformin (Synjardy), ertugliflozin/metformin (Steglatro), linagliptin/metformin (Jentadueto), saxagliptin/metformin ER (Kombiglyze XR), and sitagliptin/metformin (Janumet). A triple FDC of empagliflozin, linagliptin, and metformin ER is also available
Considerations for Use of SGLT2 Inhibitors in Combination Therapy
SGLT2 inhibitors have a unique mechanism of action that make them complementary to other currently available glucose-lowering medication classes. Up to 180 grams of glucose is filtered from the renal glomeruli of a healthy adult with normal glucose tolerance each day, and essentially all of the filtered glucose is reabsorbed and returned to the circulation. 64 Of the filtered glucose, 90% is reabsorbed into the bloodstream by the SGLT2 cotransporter, located in the S1 segment of the proximal renal tubules.64 In patients with T2DM, however, renal glucose transport is increased, as hyperglycemia leads to an upregulation of SGLT2. This results in an increase in glucose reabsorption, leading to prolongation of chronic hyperglycemia, and ultimately to exacerbation of microvascular complications. 64
SGLT2 inhibitors have shown efficacy in lowering blood glucose both as monotherapy and in combination with other glucose-lowering agents. When examining SGLT2 inhibitors plus metformin therapy combined with GLP-1 RAs, thiazolidinediones (TZDs), sulfonylureas (SUs), or insulin, all combinations showed greater net glycemic efficacy than either agent alone and meaningful reductions of A1C.16 However, the pleiotropic effects may be more significant than the glycemic benefits.16 The weight loss observed with these agents may help diminish the weight gain associated with other agents used to treat T2DM.66 For example, a study showed a weight decrease of 0.9 kg for patients taking dapagliflozin in combination with insulin compared to a 1.8 kg weight gain for those using insulin with placebo.67 Combination therapy can also minimize the need for dose escalation of other agents and can be insulin sparing.5 For example, patients taking SGLT2 inhibitors in a clinical trial did not require increased insulin doses during the study period but the placebo group had a mean insulin dose increase of 18.3 IU/day.67 Caution is recommended in the prescribing information for use of SGLT2 inhibitors with insulin or the insulin secretagogues, however, due to the potential additive risk for hypoglycemia. 31,36-38 Dose reduction is suggested to be considered to reduce the risk of hypoglycemia when used together.
Considerations for Use of DPP-4 inhibitors in Combination Therapy
The effects of GLP-1, a peptide hormone with a short plasma half-life, are diminished in T2DM. Inhibition of DPP-4, an enzyme that degrades GLP-1, leads to elevations in GLP-1 concentration and correction of the diminished effects on insulin and glucagon. DPP-4 inhibitors are becoming the most frequent drug added to metformin after initial treatment failure because of their ability to help patients achieve target A1C without hypoglycemia or weight gain, largely taking the place of SUs which have comparatively higher risks for hypoglycemia and weight gain.68 Important attributes include good overall tolerability, cardiovascular safety, and efficacy and safety with renal impairment. A low risk for contributing to hypoglycemia and weight neutrality are important when combining DPP-4 inhibitors with other agents. Combination with insulin may lead to insulin dose reductions and thus, less hypoglycemia. DPP-4 inhibitors are not recommended for use with GLP-1 RAs. Because of their related mechanisms of action, there is not significant added benefit to justify combination use. Agents with complimentary mechanisms of action, such as SGLT2 inhibitors, would be more beneficial for use with DPP-4 inhibitors.68
Combination Therapy with SGLT-2 Inhibitors + DPP-4 Inhibitors: A Review of the Data
The complementary mechanisms of action of SGLT2 inhibitors and DPP-4 inhibitors make these classes of medications desirable to use in combination to help patients meet their individualized treatment goals. Potential positive aspects of this combination when added to metformin or used when metformin is contraindicated or not tolerated include: 15
- Complementary mechanisms that target multiple pathophysiologic pathways
- Use in any stage of diabetes progression
- No increase in the risk of hypoglycemia
- Does not cause weight gain
- Many can be given as a single pill
These combination can reduce A1C more than either agents alone. 15 In phase 3 trials comparing combination therapy to either agent on its own, A1C reductions were about 1.2-1.5%, which was significantly more than either agent used independently.16 A meta-analysis of ten SGLT2 inhibitor/DPP-4 inhibitor combination studies, usually added to metformin, found significantly greater A1C reductions when compared to DPP-4 inhibitors alone (0.62%; 95% CI 0.51-0.73; P , 0.001) or SGLT2 inhibitors alone (0.32%; 95% CI 0.22-0.48; P , 0.001), and additionally found that the glucose lowering effect was greater when SGLT2 inhibitors are added to DPP-4 inhibitors versus adding a DPP-4 inhibitor to an SGLT2 inhibitor.16
The two classes do not have related adverse effects or toxicities and may actually counteract some of the others potential adverse events. Rates of genital infections, associated with SGLT2 inhibitors, were lower in combination possibly due to DPP-4 inhibitor effects on the immune system. 16 The risk of DKA may be lower with the combination because of an enlarged insulin/glucagon ratio.
Currently, there are three SGLT-2 inhibitors/DPP-4 inhibitor FDCs available (see Table 5).
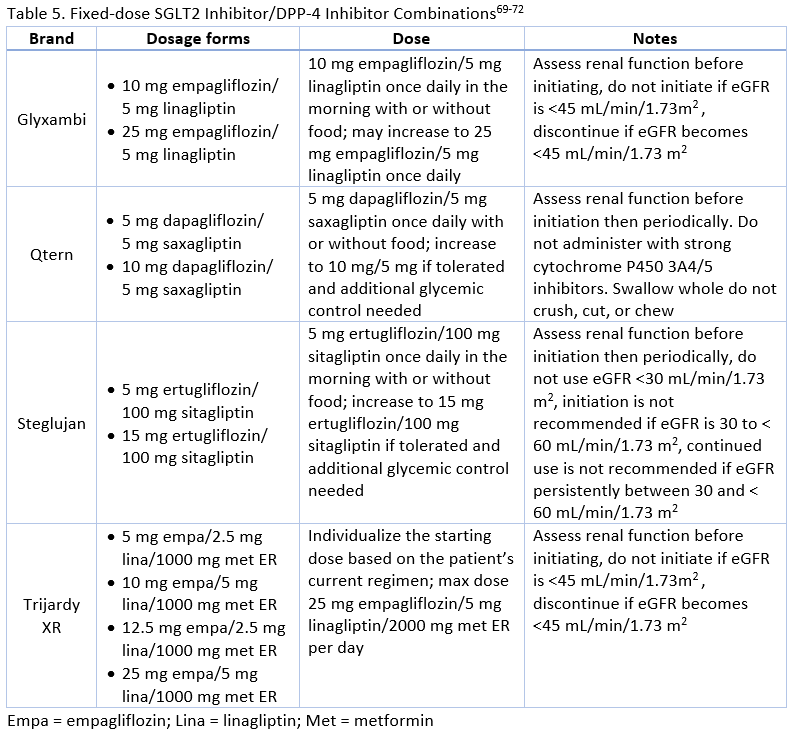
FDCs can reduce medication regimen complexity and pill burden for patients, potentially leading to better adherence.5 Studies indicate that patients are more likely to be adherent when moving from monotherapy to combination therapy when FDCs are used when compared to separate drug coadministration.5 Switching to a FDC from two separate pills increased medication adherence from 71% to 87% in one study of diabetes treatment.58 FDCs may be particularly helpful for older adults, mentally or physically impaired patients, patients with irregular schedules, or anyone less likely to adhere to complex regimens. 5 Despite these benefits, use of FDCs is low.
Numerous clinical trials have demonstrated the effects on these combinations on patients with T2DM. The results of some of these trials are summarized below.
Dapagliflozin/Saxagliptin
Rosenstock : Compared the efficacy and safety of the addition of saxagliptin 5 mg, dapagliflozin 10 mg, or dapagliflozin/saxagliptin 10/5 mg to metformin in patients who were poorly controlled.
Mathieu : Evaluated the efficacy and safety of the addition of dapagliflozin 10 mg or placebo in patients taking saxagliptin 5 mg and metformin.
Muller-Wieland : Compared the efficacy and safety of dapagliflozin 10 mg, dapagliflozin/saxagliptin 10/5 mg, or glimepiride 1 to 6 mg (titrated) added to metformin in patients with poor glycemic control.
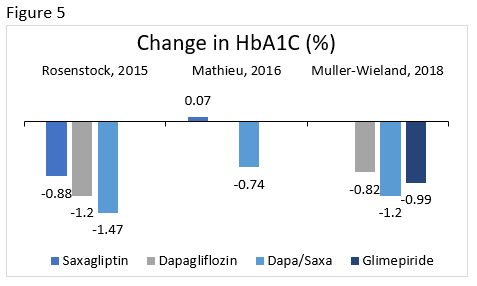 The change in A1C is illustrated for select clinical trials comparing dapagliflozin/saxagliptin to various therapies in Figure 5. Other common effects included reduction in body weight, minimal hypoglycemia, more genital infections with dapagliflozin, and overall good tolerability. 73-75
The change in A1C is illustrated for select clinical trials comparing dapagliflozin/saxagliptin to various therapies in Figure 5. Other common effects included reduction in body weight, minimal hypoglycemia, more genital infections with dapagliflozin, and overall good tolerability. 73-75
Empagliflozin/Linagliptin
Lewin : Compared the efficacy and safety of linagliptin 5 mg, empagliflozin 10 mg, empagliflozin 25 mg, empagliflozin/linagliptin 10/5 mg, and empagliflozin/linagliptin 25/5 mg treatment in patients uncontrolled with diet and exercise.
Kawamori : Evaluated the efficacy and safety of adding empagliflozin 10 mg or 25 mg to treatment in patients who were uncontrolled on linagliptin 5 mg.
Kaku : In patients not controlled on empagliflozin, this study compared the efficacy and safety of the addition of placebo or linagliptin 5 mg to treatment with empagliflozin 10 mg or empagliflozin 25 mg.
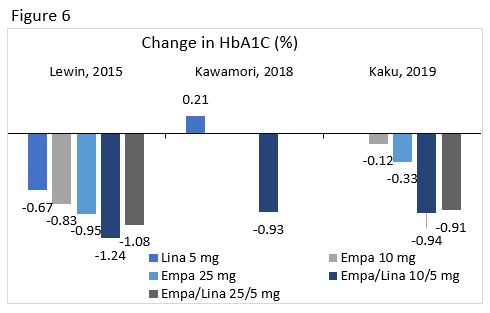 A1C changes from select clinical trials for linagliptin, empagliflozin 10 mg and 25 mg, and their combinations are seen in Figure 6. Overall, there was a low risk of hypoglycemia with this combination and patients achieved weight loss.76-78 Regimens containing empagliflozin had higher rates of genital infections.79 Reductions in SBP were seen, but these changes were not consistently significant.
A1C changes from select clinical trials for linagliptin, empagliflozin 10 mg and 25 mg, and their combinations are seen in Figure 6. Overall, there was a low risk of hypoglycemia with this combination and patients achieved weight loss.76-78 Regimens containing empagliflozin had higher rates of genital infections.79 Reductions in SBP were seen, but these changes were not consistently significant.
Ertugliflozin/sitagliptin
VERTIS SITA2 : Compared the efficacy and safety of ertugliflozin or placebo added to patients uncontrolled on sitagliptin and metformin.
VERTIS FACTORIAL : Compared the efficacy and safety of sitagliptin 100 mg, ertugliflozin 5 mg, ertugliflozin 25 mg, ertugliflozin/sitagliptin 5/100 mg, and ertugliflozin/sitagliptin 25/100 mg added to metformin in uncontrolled patients.
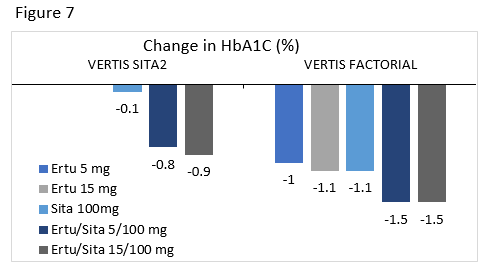 Ertugliflozin/sitagliptin is the newest of these FDCs. A1C changes from two clinical trials for this combination are seen in Figure 7.80,81 As with the other FDCs, there was a higher incidence of genital mycotic infections in groups taking ertugliflozin. Addition of ertugliflozin to sitagliptin treatment led to reductions in weight and SBP.80 There were no differences in hypoglycemia, hypovolemia related events, or urinary tract infections between groups.80,81
Ertugliflozin/sitagliptin is the newest of these FDCs. A1C changes from two clinical trials for this combination are seen in Figure 7.80,81 As with the other FDCs, there was a higher incidence of genital mycotic infections in groups taking ertugliflozin. Addition of ertugliflozin to sitagliptin treatment led to reductions in weight and SBP.80 There were no differences in hypoglycemia, hypovolemia related events, or urinary tract infections between groups.80,81
Overall, regimens with SGLT2 inhibitors had similar weight loss when compared to combinations, but had more genital infections, either as monotherapy or in combination as would be expected given their known adverse effect profile.
Conclusions
Current clinical recommendations note the importance of combination therapy to meet individualized glycemic goals. While stepwise addition of glucose-lowering therapy is generally recommended to meet individualized glycemic targets, some patients may benefit from initial combination therapy. SGLT2 inhibitors and DPP-4 inhibitors are important oral glucose-lowering therapy options that can improve glycemic control, while also minimizing hypoglycemia and weight gain.
It is also necessary to stress the importance of avoiding clinical inertia in T2DM. Addressing physician- and patient-related factors leading to inertia, including improving medication adherence, is important in achieving glycemic control. Fixed-combination products combining DPP-4 inhibitors and SGLT-2 inhibitors are available for patients who may benefit from use of medications from both classes to further simplify medication taking and therefore increase chances of good medication adherence while intensifying therapy to meet individualized treatment goals.
References
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
2. Fu AZ, Qiu Y, Davies MJ, et al. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13(8):765-769.
3. Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care. 2016;39(9):1527-1534.
4. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
5. Cersosimo E, Johnson EL, Chovanes C, Skolnik N. Initiating therapy in patients newly diagnosed with type 2 diabetes: Combination therapy vs a stepwise approach. Diabetes Obes Metab. 2018;20(3):497-507.
6. Carls G, Huynh J, Tuttle E, et al. Achievement of Glycated Hemoglobin Goals in the US Remains Unchanged Through 2014. Diabetes Ther. 2017;8(4):863-873.
7. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2019 Dec 19. doi: 10.1007/s00125-019-05039-w. [Epub ahead of print]
8. American Diabetes Association. Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020;43(Suppl. 1):S1-S224.
9. Blonde L, Raccah D, Lew E, et al. Treatment intensification in type 2 diabetes: A real-world study of 2-OAD regimens, GLP-1 RAs, or basal insulin. Diabetes Ther. 2018;9(3):1169-1184.
10. Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137-S145.
11. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(5):410-417.
12. Milligan S. Combination therapy for the improvement of long-term macrovascular and microvascular outcomes in type 2 diabetes: Rationale and evidence for early initiation. J Diabetes Complications. 2016;30(6):1177-1185.
13. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701.
14. Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors' expert forum. Diabetes Care. 2018;41(1):14-31.
15. Lingvay I. Sodium glucose cotransporter 2 and dipeptidyl peptidase-4 inhibition: Promise of a dynamic duo. Endocr Pract. 2017;23(7):831-840.
16. van Baar MJB, van Ruiten CC, Muskiet MHA, et al. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543-1556.
17. Zaccardi F, Webb DR, Htike ZZ, et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783-794.
18. Liu J, Tarasenko L, Terra SG, et al. Efficacy of ertugliflozin in monotherapy or combination therapy in patients with type 2 diabetes: A pooled analysis of placebo-controlled studies. Diab Vasc Dis Res. 2019;16(5):415-423.
19. Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372-382.
20. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
21. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
22. Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8(5):330-339.
23. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
24. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
25. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
26. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
27. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099.
28. Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018;10(2):88-89.
29. Seidu S, Kunutsor SK, Cos X, et al K; For and on behalf of Primary Care Diabetes Europe. SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: A systematic review and meta-analysis. Prim Care Diabetes. 2018;12(3):265-283.
30. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295-2306.
31. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.;2020.
32. Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749-761.
33. Jingfan Z, Ling L, Cong L, et al. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in type 2 diabetes mellitus with inadequate glycemic control on metformin: a meta-analysis. Arch Endocrinol Metab. 2019;63(5):478-486.
34. Bersoff-Matcha SJ, Chamberlain C, Cao C, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: A review of spontaneous postmarketing cases. Ann Intern Med. 2019;170(11):764-769.
35. Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687-1693.
36. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2020.
37. Farxiga [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2020.
38. Steglatro [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.;2019.
39. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.;2016.
40. Tradjenta [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2019.
41. Onglyza [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2017.
42. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.;2019.
43. Godinho R, Mega C, Teixeira-de-Lemos E, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: A "me too" or "the special one" antidiabetic class? J Diabetes Res. 2015;2015:806979.
44. Haluzík M, Mráz M, Svačina Š. Balancing benefits and risks in patients receiving incretin-based therapies: focus on cardiovascular and pancreatic side effects. Drug Saf. 2014;37(12):1003-1010.
45. Avogaro A, Delgado E, Lingvay I. When metformin is not enough: Pros and cons of SGLT2 and DPP-4 inhibitors as a second line therapy. Diabetes Metab Res Rev. 2018;34(4):e2981.
46. Matthews DR, Paldánius PM, Proot P; VERIFY study group. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394(10208):1519-1529.
47. Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401-409.
48. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20(2):427-437.
49. Lanzinger S, Schmid SM, Welp R, et al. Clinical inertia among patients with type 2 diabetes mellitus treated with DPP-4i and/or SGLT-2i. Diabetes Res Clin Pract. 2018;146:162-171.
50. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411-3417.
51. Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:2042018819844694.
52. Yam FK, Adams AG, Divine H, et al. Clinical inertia in type 2 diabetes: A retrospective analysis of pharmacist-managed diabetes care vs. usual medical care. Pharm Pract (Granada). 2013;11(4):203-10.
53. Cowart K, Sando K. Pharmacist impact on treatment intensification and Hemoglobin A(1C) in patients with type 2 diabetes mellitus at an academic health center. J Pharm Pract. 2019;32(6):648-654.
54. Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30(4):807-12.
55. Shields BM, Hattersley AT, Farmer AJ. Identifying routine clinical predictors of non-adherence to second-line therapies in type 2 diabetes: A retrospective cohort analysis in a large primary care database. Diabetes Obes Metab. 2020;22(1):59-65.
56. Brunton SA, Polonsky WH. Hot topics in primary care: medication adherence in type 2 diabetes mellitus: real-world strategies for addressing a common problem. J Fam Pract. 2017;66(4 Suppl):S46-S51.
57. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299-307.
58. Odegard PS, Capoccia K. Medication taking and diabetes: a systematic review of the literature. Diabetes Educ. 2007;33(6):1014-1029; discussion 1030-1031.
59. Marzec LN, Maddox TM. Medication adherence in patients with diabetes and dyslipidemia: associated factors and strategies for improvement. Curr Cardiol Rep. 2013;15(11):418.
60. Wardian J, Bersabe D, Duke C, Sauerwein TJ. Patient commitment and its relationship to A1C. Clin Diabetes. 2018;36(4):295-304.
61. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management Education and Support. Diabetes Educ. 2019;45(1):34-49.
62. AADE 7™ Self-Care Behaviors: American Association of Diabetes Educators (AADE) Position Statement. American Association of Diabetes Educators website. https://www.diabeteseducator.org/docs/default-source/practice/practice-resources/position-statements/aade7-self-care-behaviors-position-statement.pdf?sfvrsn=6. Accessed December 31, 2019.
63. Cho YK, Kang YM, Lee SE, et al. Efficacy and safety of combination therapy with SGLT2 and DPP4 inhibitors in the treatment of type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2018;44(5):393-401.
64. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1(2):140-151.
65. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
66. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8(8):495-502.
67. Wilding JP, Woo V, Rohwedder K, et al; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16(2):124-136.
68. Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol (Lausanne). 2019;10:389.
69. Glyxambi [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2020.
70. Qtern [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2020.
71. Steglujan [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.;2019.
72. Trijardy XR PIs [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2020.
73. Müller-Wieland D, Kellerer M, Cypryk K, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(11):2598-2607.
74. Mathieu C, Herrera Marmolejo M, González González JG, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1134-1137.
75. Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
76. Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38(3):394-402.
77. Kaku K, Haneda M, Tanaka Y, et al. Linagliptin as add-on to empagliflozin in a fixed-dose combination in Japanese patients with type 2 diabetes: Glycaemic efficacy and safety profile in a two-part, randomized, placebo-controlled trial. Diabetes Obes Metab. 2019;21(1):136-145.
78. Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: Glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;20(9):2200-2209.
79. DeFronzo RA, Lee C, Kohler S. Safety and tolerability of combinations of empagliflozin and linagliptin in patients with type 2 diabetes: Pooled data from two randomized controlled trials. Adv Ther. 2018;35(7):1009-1022.
80. Dagogo-Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20(3):530-540.
81. Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: The VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20(5):1111-1120.