
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 9: Providing Pharmaceutical Care and Products for Horses
INTRODUCTION
There are more than 7.5 million pet horses in the United States.1 Many horses also perform duties for humans—for example, they compete as athletes (eg, racehorses, show horses), have roles as service animals, or perform manual labor.
Although horses may suffer from many of the same diseases and conditions that humans do, therapies can vary significantly regarding drugs, doses, frequencies, and routes of administration. The tremendous size of equine patients often necessitates much larger doses than those used in human patients. Horses have a unique gastrointestinal (GI) tract that makes them susceptible to toxicity from drugs and substances that are not toxic in other species. Horses cannot vomit, so administration of a potentially toxic substance by the oral route can have disastrous results in horses. In addition, because horses have duties as described above, it is critical that (1) drug therapy not impair the horse’s ability to perform its job and (2) no violative drug residues cause disqualification from a competitive event.
This activity is designed to impart a working knowledge of the most common equine disease states. It is intended as a primer for pharmacists and pharmacy technicians to begin to consider the significant species-specific variations in medical therapy for horses vs humans and to learn important techniques for due diligence for prescription review, caregiver counseling, and practice tips for caring for horses.
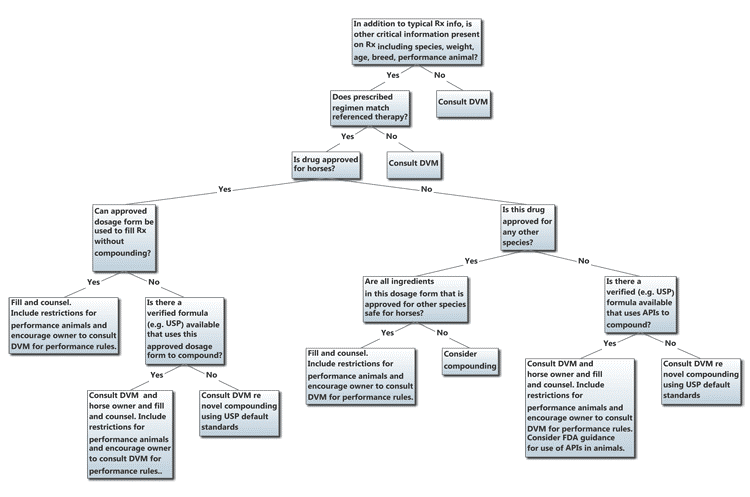
A general decision framework for evaluating and dispensing prescriptions for equine patients is outlined in Figure 1. Note that many decisions refer the pharmacist and pharmacy technician back to the prescribing veterinarian for a consultation to further clarify therapy or collaborate on a particular formulation to dispense.
Figure 1. Due Diligence for Equine Prescriptions
PITUITARY PARS INTERMEDIA DYSFUNCTION (EQUINE CUSHING’S DISEASE)
Pituitary pars intermedia dysfunction (PPID)—also known as equine Cushing’s disease—is a disorder of the pituitary gland that affects many organ systems in horses. In dogs and humans, Cushing’s disease is caused by neoplasia of the pars distalis of the pituitary gland. Equine Cushing’s disease involves the pars intermedia of the pituitary gland and is more likely to result from faulty negative feedback by dopaminergic mechanisms. In the normal equine pituitary gland, melanotropes release dopamine, which inhibits the production and release of many different pituitary hormones. In horses with PPID, the melanotropes become damaged, and dopamine inhibition is lost. Loss of dopamine negative feedback results in overproduction of proopiomelanocortin (POMC), adrenocorticotropic hormone (ACTH), melanocyte-stimulating hormones (MSHs), β endorphin, corticotropin-like intermediate lobe peptide (CLIP), lipotropins, and several other small peptides. In contrast to Cushing’s disease in humans and dogs, levels of cortisol usually are normal or low in horses, indicating that POMC derivatives other than ACTH and cortisol play an important role in disease development.
Although the precise cause of PPID is unknown, evidence suggests that oxidative stress may contribute to melanotrope damage and cell death. Histologic examination of the pituitary gland of horses with PPID revealed a 16-fold increase in the levels of oxidative stress marker 3-nitrotyrosine in the nerve terminals of the periventricular dopaminergic neurons compared with healthy adult horses.2 Lipofuscin pigment (accumulated oxidized cellular debris) also is abundant in the pituitary neurons of horses with PPID.3
Interestingly, Parkinson’s disease in humans also results from dopamine deficiency and has been noted to have a geographical distribution that heavily favors exposure to oxidative factors in agricultural areas. No distribution analysis for horses with PPID exists, but the similarity of the disease to Parkinson’s may suggest an environmental etiology.
Equine PPID occurs in middle-aged to older horses (mean age 20 years). Veterinarians generally perceive mares to be at higher risk, but this has not been supported by epidemiological review.
Likewise, although ponies and Morgan horses are thought to be over-represented, this also has not been supported by epidemiological review.4
Laminitis is the most serious clinical sign of PPID. Inflammatory and degenerative changes in the lamina of the hoof lead to chronic foot pain and lameness. Severe laminitis often necessitates euthanasia.
Other clinical signs of PPID in horses include:
- A long, wavy hair coat that fails to shed normally in spring and summer
- Lethargy and poor athletic performance
- Excessive sweating
- Weakness
- Abnormal distribution of fat in characteristic locations (supraorbital, crest of neck, rump)
- Weight loss and muscle wasting, including thinning of the topline
- Polydipsia and polyuria
Horses with PPID are prone to frequent infections (in particular, infections involving the teeth and sinuses) and delayed wound healing. Female horses may experience loss of heat cycling and infertility.
Horses with PPID that receive appropriate treatment and do not develop serious complications (eg, laminitis) can live a relatively normal life (25-30 years). The prognosis depends in large part on the extent of clinical disease at diagnosis and the owner’s commitment to treatment (including financial commitment).
Treatment Options
The goal of pharmacologic therapy for PPID is to restore dopamine negative feedback on pars intermedia production of POMC. The potent dopamine D2-receptor agonist pergolide mesylate is the drug of choice for the treatment of PPID.
Nonpharmacologic support measures are essential to the success of treatment. Routine preventive care should include dentistry, hoof care, and deworming. High-quality diets that are low in carbohydrates and consist primarily of easy-to-chew pellets are best suited for horses with PPID. Horses with PPID may have difficulty cooling themselves; it is important that they have access to ample fresh water, shade, and shelter, with body clipping and blanketing as needed. Horses ideally should have constant turnout (ie, should not be kept exclusively in stalls) with adequate shelter. If constant turnout is not possible, stall shavings should be changed frequently to avoid wet stalls from excessive urination.
Equine metabolic syndrome (EMS) has clinical signs that are very similar to those of PPID, and EMS is a consideration when ruling out PPID. EMS is a relatively new disease in equine medicine; the pathophysiology and treatment still are being worked out. Treatment currently focuses on dietary management and pharmacologic therapy with levothyroxine or metformin (or both). Pioglitazone has been considered for use in EMS and is sometimes used and velagliflozin is occasionally used (although there are no data to substantiate its use).
Pergolide Mesylate
Pergolide is a synthetic ergot derivative. It is FDA-approved for the control of clinical signs associated with PPID in horses (Prascend®).5 Pergolide is available as 1-mg oral tablets. Therapy with pergolide is initiated at a dosage of 0.002-0.004 mg/kg by mouth every 24 hours. The dose is adjusted in increments of 0.5 mg to 1 mg based on clinical response. Miniature horses and donkeys are typically started on an empirical dose of 0.5mg/ANIMAL.
Pergolide had been approved for the treatment of Parkinson’s disease in humans, but it was withdrawn from marketing in 2007 following association with valve regurgitation, valvular vegetative lesions, and cardiac lesions. These lesions have not been reported in horses treated with pergolide. During the period between when pergolide was withdrawn from marketing and Prascend® was approved in 2011, many pharmacists were approached to compound pergolide for administration to horses. Pharmacists and pharmacy technicians still may be approached about compounding custom formulations; pharmacists and pharmacy technicians should use the approved pergolide tablets—not bulk drug substance—as the source of active ingredient.6
Pharmacists and pharmacy technicians also should use verified compounding formulas for preparing oral suspensions of pergolide.7 The compounded suspension must be protected from light, stored in the refrigerator, and discarded 30 days after compounding.
Of interest to pharmacists and pharmacy technicians, the daily dose of pergolide in horses (1 mg) is similar to the total daily dose in humans (1-3 mg). The site of drug action in horses (pars intermedia of the pituitary) lies outside the blood-brain barrier; the site of drug action in humans treated for Parkinson’s disease (substantia nigra) is located in the midbrain. Thus, less drug is required in horses on a milligram per kilogram basis because the drug does not have to cross the blood-brain barrier.
Anorexia is the most common adverse effect of pergolide therapy. It usually occurs during dose escalation and may be avoided by increasing the dose gradually. Tolerance also may be improved by administering the total daily dose as 2 divided doses.
Pergolide can prolong pregnancy, result in dystocia, and decrease milk production.
Other Drugs
Cyproheptadine was used in the past to treat PPID. It is not superior to pergolide and currently is used primarily as adjunct therapy.8 When cyproheptadine is administered concurrently with pergolide, the dosage is 0.25 mg/kg by mouth every 12 to 24 hours. Because of the large number of tablets required to achieve that dose in most horses, compounding may be necessary to create an acceptable dosage form. Efficacy may not be fully apparent for 4 to 8 weeks after therapy is initiated.
Although trilostane has been suggested as a possibly effective treatment for horses with adrenal gland hyperplasia and hypercortisolemia, trilostane would not be expected to improve clinical signs associated with PPID, because inhibition of 3β-hydroxysteroid dehydrogenase does not inhibit formation of pituitary-derived hormones.
Vitex agnus castus (chasteberry) also has been suggested as a potential therapy for PPID. However, clinical evaluation in 14 horses failed to resolve clinical signs or improve diagnostic test results.9 In that study, 8 of 9 horses responded to subsequent treatment with pergolide.
Pain from laminitis typically is managed with nonsteroidal anti-inflammatory drugs (NSAIDs).
Monitoring and Follow-Up
Monitoring of horses treated for PPID includes ongoing evaluation of clinical signs and laboratory testing (plasma ACTH concentration, fasting serum insulin concentration, or both). Horses should be observed closely for signs of anorexia, infection, laminitis, non-healing wounds, and any changes that could indicate worsening of disease. A dexamethasone suppression test, measurement of fasting serum insulin concentration, or both usually are performed 4 to 8 weeks after initiation of pergolide therapy and 4 to 8 weeks after any dosage adjustment.
Safety Considerations for Humans
Caregivers with allergies to ergot derivatives should avoid contact with pergolide. Women who are pregnant or breastfeeding also should avoid contact with pergolide. Pergolide tablets have been reported to cause eye irritation, an irritating smell, or headache when they are split or crushed, so care should be taken to minimize exposure.
Client Counseling and Pharmacist/Pharmacy Technician Practice Tips:
Pituitary Pars Intermedia Dysfunction
Client Counseling
- Watch the horse for signs of long shaggy coat, weight loss, infection, abscesses, non-healing wounds, laminitis, lameness, difficulty eating, excessive water consumption, or excessive urination. Any of these can indicate that the disease is worsening or not responding to treatment.
- Herbal therapies (eg, chasteberry) have not proven to be effective and should not be used in place of medical therapy.
- Compounded pergolide oral suspension should be protected from light, stored in the refrigerator, and discarded 30 days after it is prepared.
Practice Tips for Pharmacists and Pharmacy Technicians
- Maintain inventories of pergolide for management of PPID.
- Take precautions when crushing pergolide tablets; eye irritation and headaches have been reported in humans.
- Provide medications in a sugar-free powdered vehicle (eg, psyllium) that can be administered as a top dressing on feed using calibrated scoops.
- Maintain inventories of thyroid stimulating hormone for diagnosis of PPID.
- Help veterinarians obtain diagnostic agents that are expensive or hard to find.
- Maintain inventories of horse-friendly drug administration devices (eg, 60 mL catheter tip syringes).
|
EQUINE PROTOZOAL MYELOENCEPHALITIS
Equine protozoal myeloencephalitis (EPM) is an infectious neurologic disease that affects horses in North America, Central America, and South America. It is caused primarily by the protozoan Sarcocystis neurona and less frequently by the related pathogen Neospora hughesi.10 Horses become infected with the protozoan when they ingest food or water that has been contaminated with feces from an infected opossum (the definitive host). The mode of transmission for Neospora hughesi remains uncertain.
EPM occurs most commonly in horses younger than 4 years of age (range, 2 months-19 years). No gender risk has been noted. All breeds are affected; Thoroughbreds, Standardbreds, and Quarter Horses are overrepresented.11
Although many horses in the Americas appear to be infected (based on evidence of antibodies), clinical disease is rare (< 1% of seropositive horses). In those horses, ingested sporocysts enter the bloodstream from the intestine and cross the blood-brain barrier, where they cause neurological damage. A weakened immune response subsequent to stress may increase the likelihood of infection progressing to neurological disease.
In EPM, almost any part of the central nervous system may become infected, so almost any neurologic sign is possible. Ataxia, spasticity, abnormal gait or lameness, incoordination, and weakness all are common signs. Affected horses may assume a splay-footed stance or lean against stall walls for support. Other signs of EPM include:
- Muscle atrophy along the topline or in the large muscles of the hindquarters
- Head tilt with poor balance
- Paralysis of muscles of the eyes, face, or mouth, evidenced by drooping eyes, ears, or lips
- Difficulty swallowing
- Seizures or collapse
- Abnormal sweating
- Loss of sensation along the face, neck, or body
The prognosis for EPM depends on the severity of neurological damage at time of diagnosis. Severely affected horses rarely recover fully. With mild clinical signs and early diagnosis, there is a good probability of full recovery with treatment and supportive care.
Treatment Options
Treatment early in the course of EPM is essential for preventing central nervous system damage. Antiprotozoal (anticoccidial) therapies are used to halt infection. A number of drugs, including anti-inflammatory and immunomodulatory agents, may be used as adjunctive therapy. Information about drug therapies for EPM is summarized in Table 1.
| Table 1. Drug Therapy for Equine Protozoal Myeloencephalitis |
| Drug |
Dosage |
FDA-Approved Veterinary Dosage Forms |
| Ponazuril |
Loading dose: 15 mg/kg by mouth followed by 5 mg/kg by mouth every 24 h for 28 d |
Oral paste 15% w/w (Marquis®) |
| Diclazuril |
1 mg/kg by mouth every 24 h for 28 d |
Alfalfa pellets 1.56% w/w (Protazil®) |
| Pyrimethamine/sulfadiazine |
1 mg/kg pyrimethamine and 20 mg/kg sulfadiazine by mouth every 24 h for 90-270 d |
Oral suspension (ReBalance®): sulfadiazine 250 mg/mL (as the sodium salt), pyrimethamine 12.5 mg/mL |
| Levamisole/decoquinate |
Levamisole 1 mg/kg and decoquinate 0.5 mg/kg by mouth every 24 h for 10 d |
Not available commercially as a combination product |
| Flunixin meglumine |
1.1 mg/kg by mouth every 24 h
1.1 mg/kg by intravenous injection every 24 h |
Oral paste 1500 mg/30 mL, injectable solution 50 mg/mL (Banamine®) |
| Dexamethasone |
0.1 mg/kg by mouth every 12 h for 3-7 d
0.1 mg/kg by intravenous injection every 12 h for 3-7 d |
Injectable solution as the sodium phosphate 4 mg/mL, or as the base as 2 mg/mL |
| Vitamin E |
20 U/kg by mouth every 24 h |
Powder for oral administration
10,000 U/30 g |
The triazine antiprotozoal agents ponazuril and diclazuril are FDA-approved for treatment of EPM. These drugs do not kill S. neurona; instead, they inhibit nuclear division of schizonts and microgamonts, thereby reducing populations to numbers that can be eradicated by immune responses.
Ponazuril and diclazuril are relatively expensive medications. When cost is an important consideration, many veterinarians prescribe combination therapy with pyrimethamine and sulfadiazine. An oral suspension is FDA-approved for treatment of EPM.
Nitazoxanide had been approved for treatment of EPM but was withdrawn from marketing in 2009 because of severe adverse events that included enterocolitis, anorexia, and laminitis.12
Community pharmacists and pharmacy technicians may see prescriptions for pyrimethamine or trimethoprim–sulfamethoxazole for use in horses with EPM.
Ponazuril
Ponazuril (Marquis®) is available as a 15% w/w oral paste. The usual dosage is 15 mg/kg given by mouth as a loading dose followed by 5 mg/kg by mouth every 24 hours for 28 days. Some clinicians administer ponazuril at the higher dose for the entire first week of therapy. Doses are administered with a dial-a-dose adapter calibrated by weight to deliver a 5 mg/kg dose.
Ponazuril may cause blistering of the mouth and muzzle. Caregivers should wipe the horse’s nose clean and rinse the oral cavity with water after doses are administered.
Diclazuril
Diclazuril (Protazil®) is supplied as 1.56% w/w alfalfa pellets to be administered as a top dressing on the horse’s daily grain ration, using a calibrated scoop. The pelleted dosage form facilitates administration and may be more useful for prophylaxis of recurrence. The usual dosage is 1 mg/kg every 24 hours for 28 days.
Diclazuril appears to have a wide margin of safety. No adverse events were reported in field trials.
Pyrimethamine/Sulfadiazine
The combination of pyrimethamine and sulfadiazine is available as an oral suspension (ReBalance®) containing pyrimethamine 12.5 mg/mL and sulfadiazine 250 mg/mL (as the sodium salt). The usual dosage is 4 mL per 110 lb (50 kg) of body weight every 24 hours. This is the equivalent of 1 mg/kg of pyrimethamine and 20 mg/kg of sulfadiazine daily.
The duration of therapy depends on clinical response and usually ranges from 90 days to 270 days. Combination therapy with pyrimethamine and sulfadiazine targets protozoal folate synthesis. It is now thought that folate antagonism exerts only a static effect on S. neurona; once therapy is discontinued, horses are likely to relapse quickly.13
Immediately after treatment with pyrimethamine/sulfadiazine is initiated, a sudden die-off of organisms may worsen neurologic signs (“treatment crisis”) and persist for up to 5 weeks. While treatment crisis has most commonly been associated with use of pyrimethamine/sulfadiazine, the sudden die-off of organisms and resultant worsening in neurologic signs is possible with all of the antiprotozoal agents used to treat EPM.
Adverse effects of combination therapy with pyrimethamine/sulfadiazine include bone marrow suppression, reduced appetite or anorexia, loose stools or diarrhea, and urticaria. Trimethoprim should not be administered concurrently with pyrimethamine/sulfadiazine because of competitive inhibition with pyrimethamine and also due to increased risk for bone marrow suppression.
Levamisole/Decoquinate
Combination therapy with the immune modulator levamisole and the antiprotozoal agent decoquinate is a relatively recent approach to the treatment of EPM.14 The combination is not FDA-approved for use in horses, and no product is commercially available. Many compounding pharmacies provide this combination as an oral paste or suspension, but no data are available to support the stability of these formulations.
Combination therapy with levamisole/decoquinate has been studied at a dosage of levamisole 1 mg/kg and decoquinate 0.5 mg/kg by mouth every 24 hours for 10 days.14 Levamisole is often continued for longer than the initial 10 days. No adverse events were reported in a clinical trial involving 195 horses.14
Flunixin Meglumine. The NSAID flunixin meglumine is FDA-approved for use in horses (Banamine®). It sometimes is administered during initiation of antiprotozoal therapy to provide anti-inflammatory effects. Flunixin is available as an oral paste (1500 mg/30 mL) and an injectable solution (50 mg/mL). The usual dosage is 1.1 mg/kg every 12 hours by either the oral route or injectable route.
When administered by the injectable route in horses, flunixin usually is administered intravenously. Although labeled for intramuscular use, swelling, tissue necrosis, induration, stiffness, and sweating all have been reported following intramuscular injection, and administration by this route of administration should be strongly discouraged. Flunixin must not be administered intra-arterially; the resulting central nervous system stimulation can worsen neurologic signs of EPM.
Flunixin injectable solution sometimes is administered orally. The solution is highly alkaline (pH ~9). To prevent mucosal ulceration, the solution should be diluted with an equal volume of water before the dose is administered, and the horse’s oral cavity should be rinsed well following administration.
Dexamethasone
Dexamethasone may be used as adjunctive therapy for horses with severe central nervous system involvement that are at risk of collapse from inflammatory response. It is administered by mouth or by intravenous injection at a dosage of 0.1 mg/kg every 12 hours for 3 to 7 days.
Dexamethasone is available commercially as a solution for injection (2 mg/mL). Palatable powder packets of dexamethasone no longer are commercially available but may be compounded to facilitate administration to horses.
Corticosteroids may predispose horses to laminitis.
Vitamin E
Vitamin E may be administered to horses with EPM for its antioxidant effects. No evidence supports the efficacy of vitamin E for this use, but it likely does not cause harm.
A palatable equine synthetic vitamin E powder containing 10,000 U vitamin E per 30 g powder is available from multiple manufacturers. The powder is considerably easier to administer to horses than the gel capsules designed for humans. The usual dosage is 20 U/kg by mouth every 24 hours. The dose of water soluble vitamin E is 5000 IU given by mouth once every 24 hours.
Monitoring and Follow-Up
Horses with neurological signs must be monitored closely to prevent self-injury and ensure that they are able to eat and drink normally. Most horses respond to therapy in 4 to 6 weeks. Veterinarians may prescribe stall rest for the first 1 to 2 months after treatment is initiated.
Horses treated long term with NSAIDs such as flunixin and phenylbutazone are at increased risk for gastric, colonic ulceration and renal disease. Horses on long-term NSAIDs must be monitored for feed and water intake, signs of colic, oral ulceration, abnormal stool (loose or bloody), and changes in urination.
Owners of horses treated with pyrimethamine/sulfadiazine should return to the veterinary clinic for a complete blood count at 4 weeks after initiation of therapy and every 4 weeks thereafter.
Specific rehabilitation programs may be necessary to recondition severely affected horses.
Client Counseling and Pharmacist/Pharmacy Technician Practice Tips:
Equine Protozoal Myeloencephalitis
Client Counseling
- Secure all feed sources to prevent contamination by infected opossums.
- Observe the horse closely to prevent self-injury from neurological deficits (eg, falls, seizures, collapse, stumbling).
- Exercise caution when handling neurologically unstable horses to avoid injury from a horse that falls or experiences a seizure.
- Rinse the horse’s oral cavity with ~60 mL water and wipe the nose and lips clean after medication administration to prevent irritation. Contact the veterinarian immediately if signs of mouth or nose blisters, skin rash, or colic are observed.
- Ponazuril may be given with or without food.
- Diclazuril is administered by mixing a prescribed number of calibrated scoops with the horse’s food (top dressing). Check to be sure that the entire dose of diclazuril is consumed, not left in the bottom of the feed bin.
- Shake pyrimethamine/sulfadiazine suspension well before administration.
- Do not give food or hay within 1 hour of administering pyrimethamine/sulfadiazine to ensure maximum absorption.
- In horses treated with pyrimethamine/sulfadiazine, symptoms may worsen during the first 5 weeks after therapy is initiated. Report any signs of worsening disease to the veterinarian.
- Observe horses treated with pyrimethamine/sulfadiazine for signs of bone marrow suppression (eg, bruising, bleeding, infection, fever, lack of energy, or excessive tiredness).
- Human caregivers with sulfa allergies should avoid handling sulfadiazine products.
- Administer levamisole with food to minimize GI upset.
Practice Tips for Pharmacists and Pharmacy Technicians
- Recognize signs of EPM in horses and support clients in recognizing changes that may indicate worsening of disease or drug toxicity.
- Maintain inventories of drugs used to treat equine protozoal myeloencephalitis (ponazuril, diclazuril, pyrimethamine/sulfadiazine, levamisole/decoquinate, vitamin E powder) or facilitate rapid access to help ensure immediate initiation of prescribed therapy.
- Repackage levamisole powders into patient-specific capsules or calibrated powder scoops to help caregiver achieve exact doses of powdered dosage forms.
- Be familiar with use of all devices and techniques for administering drug therapies to equine patients.
- Be able to demonstrate use of all administration devices: dial-a-dose adapter for ponazuril, calibrated scoop for diclazuril or levamisole, oral dosing syringe for pyrimethamine/sulfadiazine.
- Maintain inventories of horse-friendly drug administration devices (eg, 60 mL catheter tip syringes, dial-a-dose oral paste dispensers, calibrated powder scoops, 1-oz capacity empty gelatin capsules).
|
RHODOCOCCUS EQUI INFECTION
Rhodococcus equi is a gram-positive, facultative intracellular veterinary pathogen that preferentially infects macrophages. R. equi infection occurs almost exclusively among foals of any breed or gender younger than 1 year of age, most commonly at 1 to 3 months of age. The usual clinical expression is subacute to chronic purulent bronchopneumonia with abscess formation and suppurative lymphadenitis.15 As many as half of infected foals develop disseminated disease at distant sites, including colitis, lymphadenitis, and skin abscesses.16 R. equi pneumonia and associated complications cause significant morbidity and mortality in young foals.
Pharmacists and pharmacy technicians may recognize R. equi as thecause of significant morbidity in 17 human patients who were treated with compounded calcium gluconate injection contaminated with the organism.17 R. equi typically does not infect humans; when it does, it causes potentially fatal pulmonary disease.
R. equi is nearly ubiquitous in soil. Disease occurs primarily at large breeding farms that have a high density of mares and foals exposed to dust and airborne concentrations of R. equi. An immune deficiency is suspected in foals that develop R. equi disease (pneumonia, disseminated disease, or both) during the period when passively acquired maternal immunity begins to wane, before independent immunocompetence is fully established.
Signs of R. equi infection include cough, fever, increased respiratory rate and effort, increased heart rate, tracheal rattling, abnormal lung sounds (crackles, wheezes), and nasal discharge. Foals with disseminated disease may experience diarrhea, polysynovitis, lameness, and uveitis.
Treatment Options
Treatment for R. equi historically involved combination therapy with rifampin and a macrolide. Erythromycin was the initial macrolide of choice.18 Over the years, erythromycin was largely replaced by azithromycin or clarithromycin; both drugs have better oral absorption than erythromycin and a longer tissue half-life in bronchial fluid and macrophages. The combination of rifampin and clarithromycin has emerged as the oral therapy of choice in foals with severe disease, based on pharmacokinetic studies, ease of administration, and retrospective comparisons of efficacy.19 No prospective, double-blinded controlled studies have been performed.
Pharmacists and pharmacy technicians should be aware that most drug interaction software programs developed for medication use in humans would flag the combination of rifampin and clarithromycin. This interaction is not important in horses. Concurrent administration of rifampin may indeed decrease the bioavailability of clarithromycin in foals, most likely through inhibition of an intestinal transporter. However, the combination still is recommended to treat R. equi pneumonia, in part because of the higher clarithromycin lung concentrations achieved with concurrent rifampin administration and the relatively long postantibiotic effect.20,21
Intramuscular administration of gamithromycin recently was demonstrated to provide concentrations above the MIC90in bronchial fluid and neutrophils for 9 days, indicating that weekly administration of gamithromycin is a feasible treatment strategy.22 Weekly administration is an attractive option because it helps to decrease further stress to the foal. Other macrolides such as tulathromycin or tilmicosin have not proven to be effective for the treatment of R. equi infection,23,24 and tilmicosin is known to be cardiotoxic to horses.
The usual dosages of drug therapies for R. equi infection are listed in Table 2.
| Table 2. Drug Therapy for Rhodococcus equi Pneumonia in Foals |
| Drug |
Dosage |
| Rifampina |
5 mg/kg by mouth every 12 h |
| Clarithromycinb |
7.5 mg/kg by mouth every 12 h |
| Azithromycinb |
10 mg/kg by mouth every 24 h for 5-7 days, then decrease frequency to every 48 h |
| Erythromycinb |
As the estolate salt: 25 mg/kg by mouth every 6-8 h; as the phosphate salt: 37.5 mg/kg by mouth every 12 h |
| Gamithromycin |
6 mg/kg by intramuscular injection every 7 d |
aAdministered as combination therapy with clarithromycin or azithromycin
bAdministered as combination therapy with rifampin |
Rifampin
As part of combination therapy with a macrolide, rifampin is administered at a dosage of 5 mg/kg by mouth every 12 hours. Rifampin is not used as monotherapy for R. equi infections because of the emergence of resistance.
Rifampin approved for humans is used off-label for the treatment of R. equi infections in horses. USP has published a verified compounded preparation monograph for rifampin oral suspension 50 mg/mL.
Rifampin discolors the urine, saliva, and tears of treated animals with an orange-red tint. Contact with tinted fluids may stain clothing permanently.
Clarithromycin
As part of combination therapy with rifampin, clarithromycin is administered at a dosage of 7.5 mg/kg by mouth every 12 hours. Clarithromycin approved for humans is used off-label for the treatment of R. equi infections in horses. It is available as tablets (250 mg and 500 mg) and an oral suspension (125 mg/5 mL). Clarithromycin also is available as an extended-release tablet (500 mg), but this dosage form is not recommended because the tablets would need to be crushed for administration to foals.
Clarithromycin therapy may cause diarrhea or hyperthermia in foals. More serious GI disturbances are possible in adult horses.
Azithromycin
When azithromycin is administered as part of combination therapy with rifampin, the initial dosage is 10 mg/kg by mouth every 24 hours for the first 5 to 7 days. The frequency of administration then is reduced to every 48 hours. Azithromycin approved for humans is used off-label for the treatment of R. equi infections in horses. It is available as tablets (250 mg, 500 mg, 600 mg) and an oral suspension (200 mg/5 mL). Azithromycin also is available as a lyophilized powder (500 mg) that is reconstituted for intravenous administration. It is very important to note that compounded suspensions of azithromycin often flocculate and settle in clumps that result in erratic concentrations of drug during therapy. For prescriptions for compounded azithromycin oral suspension, both prescriber and horse owner should be informed of the dangers of using these suspensions if the content of the suspension is not consistently uniform throughout use. Like other macrolides, azithromycin may cause hyperthermia in foals.
Use of azithromycin in adult horses requires further investigation. Although short-term use in a pharmacokinetic study did not cause life-threatening adverse events, symptoms of gastrointestinal disturbance were evident, raising concerns for use in adult horses.25
Gamithromycin
Gamithromycin is an option for single-agent antimicrobial therapy. A solution for injection (150 mg/mL) that is FDA-approved for use in beef cattle (Zactran®) is used off-label for the treatment of R. equi infections in horses. The usual dosage is 6 mg/kg by intramuscular injection every 7 days. This dosing was determined by pharmacokinetic studies; clinical efficacy has not been evaluated.
Intramuscular administration of gamithromycin may cause pain and swelling at the injection site. Doses with a volume of greater than 4 mL should be split into 2 different injection sites to minimize pain and injection site reactions.
Monitoring and Follow-Up
Care for foals suffering from R. equi usually is provided on an outpatient basis. Therapy is re-evaluated 4 weeks after treatment is initiated. Sooner evaluation is indicated if the foal exhibits signs of worsening disease or intolerance to drug therapy. Monitoring for R. equi includes a physical examination, complete blood count, and serum chemistries at 4 weeks to evaluate clinical response. Once bloodwork is normalized, thoracic radiographs or ultrasound may be performed to assess resolution of lung disease. Transtracheal wash with culture and sensitivity may be indicated for foals that do not respond to initial therapy.
Veterinarians usually recommend daily rectal temperature monitoring for affected foals. Normal rectal temperature in foals ranges from 99.5°F to 102.5°F. Caregivers should contact the veterinarian for any temperature greater than 102.5°F, which may indicate a worsening of disease or iatrogenic hyperthermia. It is important to note that hyperthermia is associated with acute respiratory distress syndrome and may
potentially be fatal. Owners should be advised to keep treated animals indoors with fans during the day or provide plenty of shade if indoor confinement is not possible. Plenty of fresh drinking water must also be provided to these foals. Any signs of diarrhea should be reported to the veterinarian immediately.
From birth to 4 months of age, foals can gain as much as 2.5 to 3 lb daily. Caregivers usually are instructed to adjust the medication dose as the foal gains weight. A weight tape can be used to estimate the foal’s weight if a scale is not available. The foal’s girth is measured with the tape just behind the elbows (heart girth). The following formulas are used to determine the correct dose:
- For foals 7-28 days old: [heart girth (inches) – 25] ÷ 0.07 = weight in pounds
- For foals 28-90 days old: add 10% to the formula for foals 7-28 days old
Weight in pounds should be divided by 2.2 to determine the per-kilogram dose.
Prognosis is good for treated foals with infections that are susceptible to macrolide therapy. Prognosis is poor for foals with macrolide-resistant infections or uveitis. Prognosis is grave for foals with intraabdominal abscesses.26
Client Counseling and Pharmacist/Pharmacy Technician Practice Tips:
Rhodococcus equi Infection
Client Counseling
- Track weight changes carefully to allow for accurate dose adjustments.
- Medication doses can be administered by crushing tablets or emptying capsules and mixing the powder with water or feed. Observe foals to ensure the entire dose is consumed.
- Adult horses should not be permitted to eat feed mixed with erythromycin, azithromycin, or clarithromycin; these medications may decimate the cecal flora of adult horses and cause colic.
- Monitor foals treated with erythromycin for iatrogenic hyperthermia.
- Rifampin will stain skin and clothing upon contact. Rifampin also will tint the tears, saliva, and urine of treated horses an orange-red color; this tint should not be mistaken for blood in these fluids.
- Pick up and discard the feces of infected foals to prevent reinfection of the foal or ingestion by the mare (organisms and drugs are eliminated in the feces).
- Contact the veterinarian immediately if the foal exhibits signs of diarrhea.
Practice Tips for Pharmacists and Pharmacy Technicians
- Maintain adequate inventories of azithromycin, clarithromycin, and rifampin for treating foals infected with R. equi.
- Identify verified compounding formulas for medications that either (1) are not available commercially in liquid formulations or (2) are not available in sufficiently concentrated liquid formulations. Drug doses are increased as the foal gains weight, and liquid dosage forms can be adjusted more easily.
- Provide foal-friendly administration devices for liquid medications (eg, catheter tip syringes).
- Demonstrate the use of weight tape and calculations based on foal age.
- Provide dosage adjustment charts indicating how much medication to administer as the foal gains weight.
|
EQUINE RECURRENT UVEITIS
Equine recurrent uveitis (ERU)—also known as moon blindness, iridocyclitis, and periodic ophthalmia—is the most common cause of blindness in horses. It is reported to affect approximately 25% of horses in the United States, but field investigations indicate that only 1% to 2% of horses have disease serious enough to result in vision loss.27,28
ERU occurs in horses of all ages, genders, and breeds. Appaloosas are 8.3 times more likely to experience ERU; Draft breeds and Warmbloods also are represented. ERU is classified as insidious (genetic predisposition in Appaloosas) or classicin presentation. Leptospirosis caused by highly invasive bacteria of the genus Leptospira can precipitate uveitis or worsen existing disease.
ERU is thought to result from an immune-mediated attack on components of the anterior chamber, leading to inflammation and (eventually) irreversible damage to the eye (eg, cataract, glaucoma, retinal detachment). Clinical signs include ocular pain manifested by tearing, blepharospasm (squinting), corneal edema, corneal ulcerations from self-trauma (rubbing painful eye against rough surface), and aqueous flare (haziness in anterior chamber). Most horses with ERU experience cyclic episodes of inflammation; miosis is the classic sign of an active episode.
The visual prognosis for horses with ERU is described as poor. One long-term study revealed complete blindness in 20% of horses and blindness in at least one eye in 56% of horses.29
Secondary glaucoma is common in horses with ERU (21% in Appaloosas).29 Fungal infection of corneal ulcers also is a potential complication of ERU.
Treatment Options
Treatment goals for ERU are to decrease inflammation and control pain. Almost all of the topical ocular therapies used to manage pain and inflammation in equine uveitis are labeled for use in humans only. Many also are too expensive for veterinarians to stock routinely; consequently, caregivers often need to have prescriptions filled at community pharmacies.
To facilitate administration of ophthalmic solutions during initial treatment of severe episodes of ERU, veterinarians may implant a subcutaneous catheter (subpalpebral lavage catheter, or SPL) communicating beneath the upper eyelid to a catheter port distal to the eye. Medication can be injected easily into the distal port and then pushed to the ocular surface using air to flush the catheter (not saline or another liquid as this will dilute the medication and wash it out of the eye). When pharmacists or pharmacy technicians receive prescriptions for administration of ophthalmic medications “through the SPL catheter,” they might consider repackaging ophthalmic solutions into multidose injection vials for more accurate and efficient removal of doses. Due to the volume capacity of the equine conjunctival sac, doses administered by SPLs are usually 0.2 mL or less.
Because of the size and shape of the equine eye, many horse owners find it easier to administer ophthalmic ointments and gels on an outpatient basis. Not all ophthalmic solutions and suspensions are available as ointments, so pharmacists and pharmacy technicians often are approached to compound more viscous ophthalmic dosage forms for use in horses. Ophthalmic dosage forms are considered to be sterile preparations and must be prepared according to USP Chapter <797> Pharmaceutical Compounding-Sterile Preparations.
Topical Anti-Inflammatory Agents
The topical anti-inflammatory agents used most commonly for the treatment of ERU are (Table 3):
- Prednisolone acetate 1% ophthalmic suspension
- Dexamethasone sodium phosphate ophthalmic solution or suspension, 0.05% and 0.1%
- Flurbiprofen 0.03% ophthalmic solution
- Diclofenac 0.1% ophthalmic solution
- Bromfenac 0.09% ophthalmic solution
All are administered to the affected eye every 1 hour to every 6 hours.
Prednisolone and dexamethasone exhibit potent anti-inflammatory activity with excellent ocular penetration. Treatment with prednisolone or dexamethasone can predispose the horse to developing fungal keratitis (corneal ulcer). Use of either drug is contraindicated when corneal ulcer or infection is present.
| Table 3. Topical Anti-Inflammatory Therapy for Equine Recurrent Uveitis |
| Drug |
Ophthalmic Dosage Form |
Frequency of Administration |
Anti-Inflammatory Activity |
Ocular Penetration |
| Prednisolone acetate |
1% suspension |
Every 1-6 h |
Potent |
Excellent |
| Dexamethasone sodium phosphate |
0.05%-0.1% solution or suspension |
Every 1-6 h |
Potent |
Excellent |
| Flurbiprofen |
0.03% solution |
Every 1-6 h |
Good |
Good |
| Diclofenac |
0.1% solution |
Every 1-6 h |
Good |
Good |
| Bromfenac |
0.09% solution |
Every 1-6 h |
Good |
Good |
Flurbiprofen, diclofenac, and bromfenac all exhibit good anti-inflammatory activity with good ocular penetration. All may delay corneal healing and epithelialization. It is possible to compound an ophthalmic gel for use in horses from either diclofenac solution or bromfenac solution.
Cyclosporine A
The strong immunosuppressant agent cyclosporine A (Table 4) is available as a 0.2% ophthalmic ointment approved for use in dogs (Optimmune®) that is used off-label for the treatment of ERU. A 2% ophthalmic emulsion is a commonly compounded formulation. Cyclosporine also is available on an investigational basis as an ocular implant.
| Table 4. Other Drug Therapies for Equine Recurrent Uveitis |
| Drug |
Dosage Form |
Dosage |
| Cyclosporine A |
Ophthalmic ointment 0.2%
Ophthalmic emulsion 2% |
Apply to the affected eye every 6-12 h |
| Atropine sulfate |
Ophthalmic solution 1%
Ophthalmic ointment 1% |
Apply to the affected eye every 6-48 h |
| Phenylephrine |
Ophthalmic solution 10% |
Apply to the affected eye every 6-12 h |
| Flunixin meglumine |
Oral paste: 1500 mg/30 mL
Injectable solution: 50 mg/mL* |
0.5 mg/kg by mouth every 12 h for 5 d, then 0.25 mg/kg every 12 h |
| Phenylbutazone |
Tablets: 1 g
Calibrated scoop powders
Oral paste: 6 g/syringe or 12 g/syringe
Solution for injection: 200 mg/mL* |
2.2 mg/kg by mouth every 12-24 h; 4.4 mg/kg by mouth every 24 h not more than 5 consecutive d |
| Prednisolone |
Tablets: 5 mg, 20 mg |
1 mg/kg by mouth every 24 h for 4 d, then taper by 25% in 4-day intervals until drug is discontinued |
| Voriconazole |
Lyophilized powder reconstituted to 10 mg/mL solution |
0.2 mL applied to the affected eye every 1-2 h, then every 6 h |
| *Administered into the oral cavity |
Cyclosporine is administered to the affected eye every 6 to 12 hours. It has relatively poor topical penetration and a weak anti-inflammatory effect when applied topically.
Atropine Sulfate
Atropine sulfate (Table 4) is available as a 1% ophthalmic solution and a 1% ophthalmic ointment approved for humans. It is used for its cycloplegic and mydriatic properties to relieve pain associated with ciliary spasm and minimize synechia formation. Atropine is administered to the affected eye every 6 to 48 hours.
Atropine may decrease gut motility and predispose the horse to colic. Caregivers should monitor treated horses for signs of ileus (decreased gut motility).
Atropine may be used to increase uveal-scleral outflow in horses that develop glaucoma secondary to uveitis.
Phenylephrine
Phenylephrine 10% ophthalmic solution (Table 4) may be used in combination with atropine as a mydriatic to relieve pain associated with ciliary spasm. It is administered to the affected eye every 6 to 12 hours.30
Oral Nonsteroidal Anti-Inflammatory Drugs
The NSAIDs flunixin meglumine and phenylbutazone (Table 4) may be administered orally to horses for the treatment of ocular pain caused by ERU.
As discussed in the section on equine protozoal myeloencephalitis, flunixin is FDA-approved for use in horses (Banamine®), available as both an oral paste (1500 mg/30 mL) and an injectable solution (50 mg/mL). When used for the treatment of ocular pain, flunixin is administered by mouth at a dosage of 0.5 mg/kg every 12 hours for 5 days; after 5 days, the dose is reduced to 0.25 mg/kg. Horses treated with flunixin should receive concurrent gastroprotective therapy (eg, omeprazole). If the injectable solution is administered orally, the horse’s oral cavity should be rinsed with 60 mL of water after the dose is administered to prevent oral ulcerations.
Phenylbutazone is available for veterinary use as tablets (1 g), calibrated scoop powders, oral paste (6 g or 12 g per syringe), and solution for injection (200 mg/mL). The usual oral dosage is 4.4 mg/kg every 12 hours. Phenylbutazone is considered to be less effective than flunixin for the treatment of ocular pain.
Oral Prednisolone
Prednisolone (Table 4) is used instead of prednisone in horses because they do not convert prednisone to prednisolone efficiently. Systemic administration of prednisolone is considered to be a therapy of last resort for the treatment of ERU.
The usual dosage of oral prednisolone is 1 mg/kg every 24 hours for 4 days. The dose then should be tapered by 25% in 4-day intervals until it is discontinued. Concurrent administration of a gastroprotective agent is recommended.
Systemic corticosteroids predispose horses to laminitis, so caregivers should be observant for any signs of laminitis.
Voriconazole
Voriconazole (Table 4) is a triazole antifungal medication approved for humans. It is used to treat fungal infections of corneal ulcers. Fungal keratitis is a medical emergency in horses; immediate initiation of therapy is critical for preventing enucleation (loss of the eye). In addition to voriconazole, compounded miconazole 1% ophthalmic solution and natamycin ophthalmic suspension may also be used for surface corneal fungal infections depending on the culture and sensitivity results.
Voriconazole is available as a lyophilized powder that is reconstituted to an injectable solution (10 mg/mL). The usual dosage for the treatment of fungal keratitis in horses is 0.2 mL administered to the affected eye every 1 to 2 hours for 3 to 5 days or until corneal melting (keratolysis) is arrested, then every 6 hours. The reconstituted solution should be stored in the refrigerator and discarded after 28 days.31
Monitoring and Follow-Up
Horses with ERU should be reevaluated by a veterinarian 10 to 14 days after initiation of therapy. Caregivers must observe horses closely for signs of clinical response. If there is any indication that the condition could be worsening, the horse should be evaluated by a veterinarian immediately.
Horses treated long term with NSAIDs such as flunixin and phenylbutazone are at increased risk for gastric, colonic ulceration, and renal disease. Horses on long-term NSAIDs must be monitored for feed and water intake, signs of colic, oral ulceration, abnormal stool (loose or bloody), and changes in urination.
Safety Considerations for Humans
Human caregivers should use caution when exposed to body fluids of horses with leptospirosis. Infection is transmitted between species by contact with infected body fluids (commonly urine) or contaminated water or soil. Leptospira bacteria can enter the body through even minor skin lesions. Clinical disease in humans ranges from mild to severe and can be fatal. Leptospira infection can cause severe adverse effects to both mother and fetus in pregnant women.
Client Counseling and Pharmacist/Pharmacy Technician Practice Tips:
Equine Recurrent Uveitis
Client Counseling
- Keep the horse quiet and away from other horses, tree branches, and pasture debris until the ERU episode resolves. Ideally, the horse should be confined to a small paddock or large box stall.
- Daily exercise should consist of not more than 15 to 30 minutes of hand walking only, several times a day.
- Horses treated with atropine should not be exposed to bright, direct sunlight. Atropine causes the pupil to dilate.
- Horses treated with atropine should be monitored for fecal output (6-8 stools daily) and signs of colic (abdominal discomfort, looking and kicking at abdomen, decreased stool output, decreased appetite).
- Administer eye medications sequentially (one after the other). Wait at least 5 minutes between medications.
- Shake ophthalmic suspensions well before administration.
- Avoid touching medication bottles or tube tips to the surface of the horse’s eye to avoid contamination.
- If a subpalpebral lavage catheter is used to administer drugs, use a new needle and syringe for each dose to avoid contamination. Flush the line with air (not saline) to avoid diluting medications.
Practice Tips for Pharmacists and Pharmacy Technicians
- Identify verified compounding formulas to prepare ophthalmic ointments and gels for the treatment of ERU that are not commercially available.
- Maintain inventories of medications used to treat ERU.
- Maintain inventories of voriconazole for treatment of potential fungal complications of ERU. Immediate access by veterinarians and horse owners is critical for avoiding enucleation (loss of the eye).
- Be familiar with subpalpebral lavage administration catheters and able to counsel caregivers on appropriate use and maintenance. Ophthalmic solutions administered via subpalpebral lavage are best dispensed in multidose sterile vials to be withdrawn with sterile syringes and needles.
|
EQUINE GLAUCOMA
Equine glaucoma is similar to glaucoma in humans: increased intraocular pressure (IOP) results in progressive deterioration of the optic nerve, leading to blindness. Normal IOP in horses ranges from 15 mm Hg to 30 mm Hg. Horses have an extensive unconventional outflow to direct aqueous humor out of the eye (uveoscleral pathways). Changes in the iridocorneal angle—the cause of primary glaucoma in humans—are not likely to produce glaucoma in horses. Horses are most likely to develop glaucoma secondary to uveitis.
Glaucoma is caused by either overproduction of aqueous humor or decrease in outflow of aqueous humor from the anterior chamber. In horses, decrease in outflow is more likely. There are several different mechanisms by which uveitis can obstruct flow of aqueous humor from its production by the ciliary body, through the pupil, into the anterior chamber, and then out of the iridocorneal angle:
- Formation of synechiae (tissue adhesion)
- Formation of fibrovascular membranes
- Blockage of outflow mechanisms by cellular debris
Although there are no breed, age, or gender associations with equine glaucoma, horses genetically predisposed to uveitis are more likely to develop glaucoma. As discussed in the section on ERU, Appaloosas are 8 times more likely than other breeds to suffer from recurrent uveitis; 21% of Appaloosas that develop recurrent uveitis are likely to experience glaucoma as a complication. Other breeds predisposed to uveitis (and thus to glaucoma as a complication) are Draft breeds and Warmbloods.
The only visible clinical sign of glaucoma is buphthalmos (an enlarged eye). Slight mydriasis may also be present.
The prognosis for equine glaucoma is guarded, even with treatment. Progressive vision loss is likely. Fortunately, horses with loss of vision in one eye (or loss of one eye) can compensate and lead normal lives with attentive and committed caregivers.
Treatment Options
No medications are approved for the treatment of glaucoma in veterinary patients. Drugs approved for humans—aimed at decreasing production or increasing outflow of aqueous humor—are used off-label for equine glaucoma. The drugs employed most commonly are topical β blockers and topical carbonic anhydrase inhibitors (Table 5).
| Table 5. Drug Therapy for Equine Glaucoma |
| Drug |
Dosage |
| Timolol maleate 0.5% ophthalmic solution |
2 drops every 12 h |
| Brinzolamide 1% ophthalmic solution |
2 drops every 12-24 h |
| Dorzolamide 2% ophthalmic solution |
2 drops every 8-12 h |
Prostaglandins (eg, latanoprost) are not recommended for use in horses. Prostaglandins are pro-inflammatory, and treatment for equine glaucoma is directed at correcting inflammation caused by uveitis. Parasympathomimetic agents such as pilocarpine and demecarium bromide also are not recommended for the treatment of equine glaucoma; they cause miosis and ocular irritation and they increase IOP significantly. Adrenergic receptor agonists (eg, brimonidine) are used widely in humans to lower IOP and may be useful in horses; however, further investigation of the safety and efficacy of these agents in normal horses and horses with glaucoma is warranted.
Beta Blockers
Timolol maleate 0.5% ophthalmic solution is the topical β blocker used most commonly for the treatment of equine glaucoma. The 0.25% ophthalmic solution has not been shown to be effective in veterinary patients. The usual dosage is 2 drops in the affected eye every 12 hours.
Gel-forming solutions of timolol have not been evaluated in horses but may be of benefit. Although it is possible that gel-forming solutions would be more efficacious in horses, it is likely that administration every 12 hours still would be required.
Carbonic Anhydrase Inhibitors
Dorzolamide 2% ophthalmic solution and brinzolamide 1% ophthalmic solution are the topical carbonic anhydrase inhibitors used most commonly for the treatment of equine glaucoma. Either drug may be compounded into an ophthalmic gel by incorporating the solution into hydrogels.
Brinzolamide appears to be twice as effective as dorzolamide in lowering equine IOP.32 The usual dosage is 2 drops in the affected eye every 12 to 24 hours.
The usual dosage of dorzolamide is 2 drops in the affected eye every 8 to 12 hours. It may cause more stinging on application than brinzolamide.
Monitoring and Follow-Up
Caregivers should observe horses closely for any changes in eye appearance. Caregivers also should watch for squinting or eye discharge.Horses that develop any of these signs should be evaluated by a veterinarian.
Dorzolamide and brinzolamide are sulfonamide drugs. Caregivers with sulfa allergies should avoid contact with both drugs.
Client Counseling and Pharmacist/Pharmacy Technician Practice Tips:
Equine Glaucoma
Client Counseling
- Contact the veterinarian if you observe any changes in the appearance of the horse’s eye.
- Contact the veterinarian if the horse begins to squint or you notice discharge from the eye.
- Avoid touching the medication dropper tip to the surface of the horse’s eye.
- Brinzolamide should be stored in the refrigerator.
- Be aware that dorzolamide may cause stinging when applied.
- Avoid contact with dorzolamide and brinzolamide if you are allergic to sulfa drugs.
Practice Tips for Pharmacists and Pharmacy Technicians
- Recognize signs of glaucoma in horses.
- Identify verified compounding formulas for converting ophthalmic solutions into ophthalmic gels.
|
REFERENCES
- American Pet Products Association. Pet industry market size & ownership statistics. http://www.americanpetproducts.org/press_industrytrends.asp. Accessed November 7, 2019.
- McFarlane D, Dybdal N, Donaldson MT, et al. Nitration and increased alphasynuclein expression associated with dopaminergic neurodegeneration in equine pituitary pars intermedia dysfunction. J Neuroendocrinol. 2005;17:73-80.
- Glover CM, Miller LM, Dybdal NO, et al. Extra-pituitary and pituitary pathological findings in horses with pituitary pars intermedia dysfunction: a retrospective study. J Equine Vet Sci. 2009;29:146-153.
- McGowan TW, Pinchbeck GP, McGowan CM. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet J. 2013;45(1):74-79.
- Prascend [package insert]. St. Joseph, MO: Boehringer Ingelheim Vetmedica, Inc; 2011. https://www.prascend.com/prescribe.html. Accessed November 7, 2019.
- FDA Center for Veterinary Medicine. Prascend (pergolide mesylate) tablets—veterinarians. Issued April 20, 2012. Reissued March 2, 2016. Available at: http://www.fda.gov/animalveterinary/safetyhealth/productsafetyinformation/ucm277207.htm. Accessed November 7, 2019.
- Davis JL, Kirk LM, Davidson GS, et al. Effects of compounding and storage conditions on stability of pergolide mesylate. J Am Vet Med Assoc. 2009;234(3):385-389.
- Donaldson MT, LaMonte BH, Morresey P, et al. Treatment with pergolide or cyproheptadine of pituitary pars intermedia dysfunction (equine Cushing’s disease). J Vet Intern Med. 2002;16(6):742-746.
- Beech J, Donaldson MT, Lindborg S. Comparison of Vitex agnus castus extract and pergolide in treatment of equine Cushing’s syndrome. In: Proceedings from the 48th Annual Convention of the American Association of Equine Practitioners; 2002.https://aaep.org/horsehealth/equine-endocrinology-cushings-disease-and-metabolic-syndrome. Accessed November 7, 2019.
- MacKay RJ, Granstrom DE, Saville WJ, et al. Equine protozoal myeloencephalitis. Vet Clin North Am Equine Pract. 2000;16(3):405-425.
- Fayer R, Mayhew IG, Baird JD, et al. Epidemiology of equine protozoal myeloencephalitis in North America based on histologically confirmed cases. J Vet Intern Med. 1990;4(2):54-57.
- Withdrawal of approval of new animal drug applications; ketamine; S-methoprene; nitazoxanide. Fed Regist. 2009;74(139):36241.
- Kane E. Equine protozoal myeloencephalitis: Etiology, diagnosis and treatment. DVM360 Magazine. October 1, 2011. https://veterinarynews.dvm360.com/equine-protozoal-myeloencephalitis-etiology-diagnosis-and-treatment?id=&sk=&date=&pageID=6. Accessed September 16, 2019.
- Ellison SP, Lindsay DS. Decoquinate combined with levamisole reduce the clinical signs and serum SAG 1, 5, 6 antibodies in horses with suspected equine protozoal myeloencephalitis. Int J Appl Res Vet Med. 2012;10(1):1-7.
- Yager JA. The pathogenesis of Rhodococcus equi pneumonia in foals. Vet Microbiol. 1987;14(3):225-232.
- Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4(1):20-34.
- District court enters permanent injunction against Texas pharmacy and senior executives to prevent distribution of adulterated and misbranded drugs [news release]. Washington, DC: U.S. Department of Justice; March 11, 2015. http://www.justice.gov/opa/pr/district-court-enters-permanent-injunction-against-texas-pharmacy-and-senior-executives. Accessed November 7, 2019.
- Giguere S, Cohen ND, Chaffin MK, et al. Diagnosis, treatment, control, and prevention of infections caused by Rhodococcus equiin foals. J Vet Intern Med. 2011;25:1209-1220.
- Giguere S, Jacks S, Roberts GD, et al. Retrospective comparison of azithromycin, clarithromycin, and erythromycin for the treatment of foals with Rhodococcus equiJ Vet Intern Med. 2004;18:568-573.
- Prescott JF, Nicholson VM. The effects of combinations of selected antibiotics on the growth of Corynebacterium equi. J Vet Pharmacol Ther. 1984;7:61-64.
- Hillidge CJ. Use of erythromycin-rifampin combination in treatment of Rhodococcus equiVet Microbiol. 1987;14:337-342.
- Berghaus LJ, Giguere S, Sturgill TL, et al. Plasma pharmacokinetics, pulmonary distribution and in vitro activity of gamithromycin in foals. J Vet Pharmacol Ther. 2012;35:59-66.
- Carlson KL, Kuskie KR, Chaffin MK, et al. Antimicrobial activity of tulathromycin and 14 other antimicrobials against virulent Rhodococcus equiin vitro. Vet Ther. 2010;11:E1-9.
- Womble A, Giguere S, Murthy YV, et al. Pulmonary disposition of tilmicosin in foals and in vitro activity against Rhodococcus equiand other common equine bacterial pathogens. J Vet Pharmacol Ther. 2006;29:561-568.
- Leclere M, Magdesian KG, Cole CA, et al. Pharmacokinetics and preliminary safety evaluation of azithromycin in adult horses. J Vet Pharmacol Ther. 2012;35(6):541-549.
- Cohen ND. Rhodococcus equifoal pneumonia. Vet Clin North Am Equine Pract. 2014;30(3):609-622.
- Schwink KL. Equine uveitis. Vet Clin North Am Equine Pract. 1992;8(3):557-574.
- Dwyer AE, Crockett RS, Kalsow CM. Association of leptospiral seroreactivity and breed with uveitis and blindness in horses: 372 cases (1986-1993). J Am Vet Med Assoc. 1995;207:1327-1331.
- Dwyer AE, Kalsow CM. Visual prognosis in horses with uveitis. Paper presented at: American Society of Veterinary Ophthalmology Annual Meeting; March 14, 1998; Chicago, IL.
- Gilger BC, Deeg C. Equine recurrent uveitis. In: Gilger BC. Equine Ophthalmology. 2nd ed. Maryland Heights, MO: Elsevier Saunders; 2011:317-349.
- Clode AB, Davis JL, Salmon J, et al. Evaluation of concentration of voriconazole in aqueous humor after topical and oral administration in horses. Am J Vet Res. 2006;67(2):296-301.
- Germann SE, Matheis FL, Rampazzo A, et al. Effects of topical administration of 1% brinzolamide on intraocular pressure in clinically normal horses. Equine Vet J. 2008;40(7):662-665.
Back to Top