
Expired activity
Please go to the PowerPak
homepage and select a course.
Introduction: Pharmacist’s Expanding Role in HIV Prevention
Addressing the national call to action to reduce the incidence of human immunodeficiency virus (HIV) infection—especially in high-risk, yet underserved populations—Colorado has proactively led the way as one of the first states to allow pharmacists, via a statewide protocol, to prescribe HIV preexposure prophylaxis (PrEP) and postexposure prophylaxis (PEP) for appropriately identified candidates. Pioneering legislation—Colorado (CO) House Bill 20-1061 and subsequent authorization under CO State Board of Pharmacy Rule 17 Appendix C (SBOP Rule 17)1,2—has made the expanded scope of pharmacy practice possible. This continuing education and training monograph will address the legislation’s provisions and regulations and provide a foundation for HIV prevention services: current clinical guidelines for PrEP and PEP provision, key pharmacologic considerations, pharmacist/patient communication, and practice-management strategies. Rule 17 specifies that all HIV-prevention care will be provided in accordance with current guidelines of the US Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force (USPSTF). In some instances, however, Colorado requirements may differ. These differences will be noted throughout this educational activity. Participants are also reminded to reference applicable regulations pertaining to all statewide protocols in Rule 17.2
HIV Epidemiology and the Burden of Disease
Approximately 1.2 million people in the United States are living with HIV today, with 1 in 7 HIV-infected individuals unaware of their infection. Latest estimates from the CDC indicate that there were 36,400 new HIV infections in the United States in 2018. Populations including men who have sex with men (MSM), transgender persons, and black and Hispanic/Latinx individuals continue to be disproportionally affected. While new annual infections in the United States have been reduced by more than two thirds since the mid-1980s when HIV initially emerged, black and Hispanic/Latinx populations are among those that continue to have high rates of undiagnosed infections (Figure 1).3,4
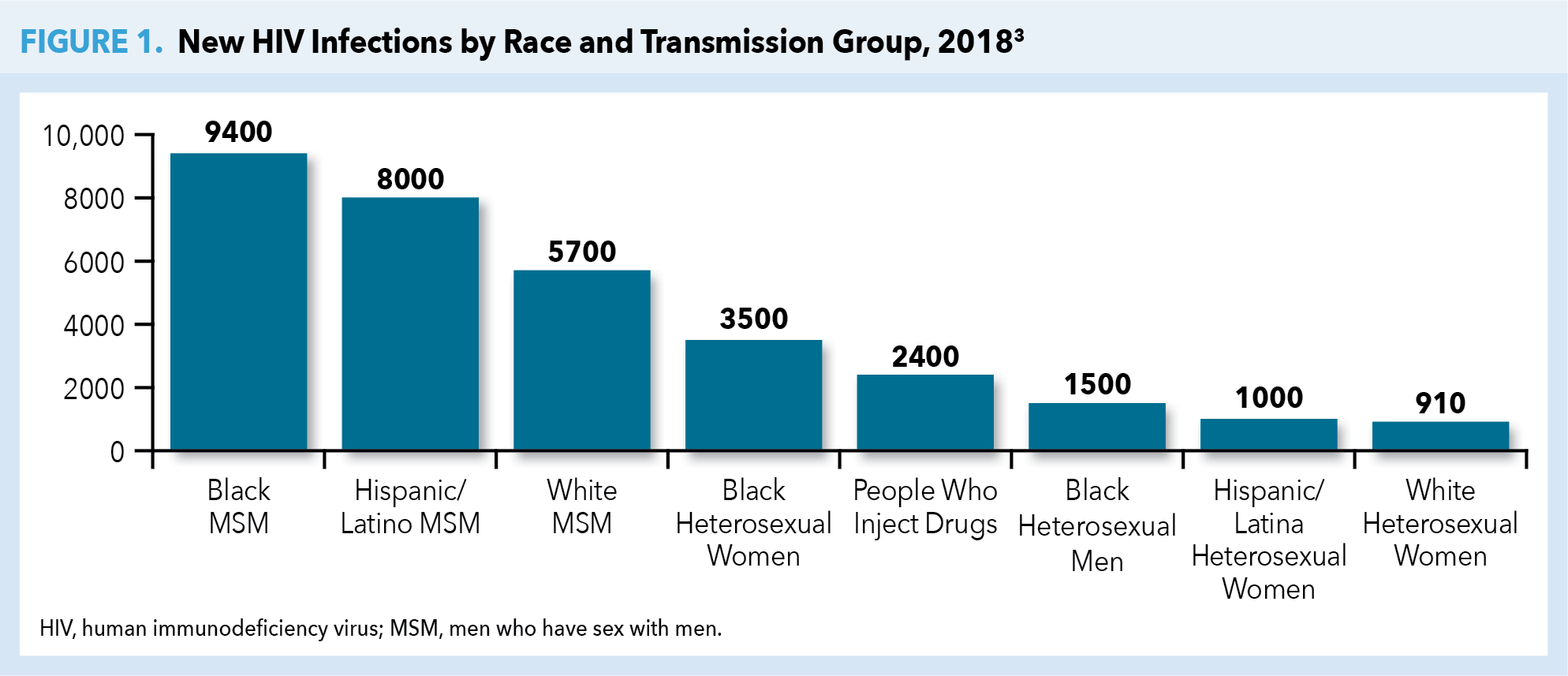
The burden of HIV has often been measured by the number of individuals dying from acquired immunodeficiency syndrome (AIDS)-related complications. Although on an international level, HIV/AIDS remains among the leading fatal infectious diseases, AIDS-related deaths in the United States have dramatically dropped because of advances in HIV treatment and management.5,6 Nonetheless, the United States government continues to spend more than $20 billion annually in direct health expenditures related to HIV care and prevention. This underscores HIV as a persistent public health threat despite greater disease awareness, highly effective antiretroviral therapy (ART), and proven prevention strategies and tactics.7
The advent and continual evolution of ART has allowed people with HIV (PWH; or people living with HIV [PLWH]) to enjoy longer, healthier lives through reduction in HIV-related morbidity and mortality at all stages of HIV infection.8 ART has also given rise to modalities designed to limit HIV acquisition in HIV-negative individuals—notably, specific antiretroviral drug combinations used as PrEP and PEP. PrEP is prescribed for HIV-negative people who are at high ongoing risk of HIV exposure, whereas PEP is prescribed for HIV-negative people who have had a single high-risk exposure.9
Despite PrEP demonstrating efficacy in reducing HIV transmission rates by as much as 74% to 99% when taken daily (depending on mode of transmission—sexual or injection drug use [IDU]), fewer than 1 in 4 potential candidates receive PrEP.10-12 High risk populations with the lowest rates of PrEP uptake include young MSM, racial or ethnic minority groups, and individuals living in the South or Southeast regions of the United States. Without preventive efforts, 1 in 2 black MSM and 1 in 5 Hispanic/Latino MSM will contract HIV in their lifetimes.13,14 The majority of PrEP uptake (75%) has been among white gay and bisexual men, predominantly those living in the Northeast or on the West Coast.15 As of 2017, the PrEP-to-need ratio (ie, the number of PrEP users divided by new HIV diagnoses) was lowest among individuals age ≤24 years or ≥55 years. Geographically, lowest ratios were seen throughout the South, despite 52% of new HIV diagnoses in the United States being attributable to this region (Figure 2).17
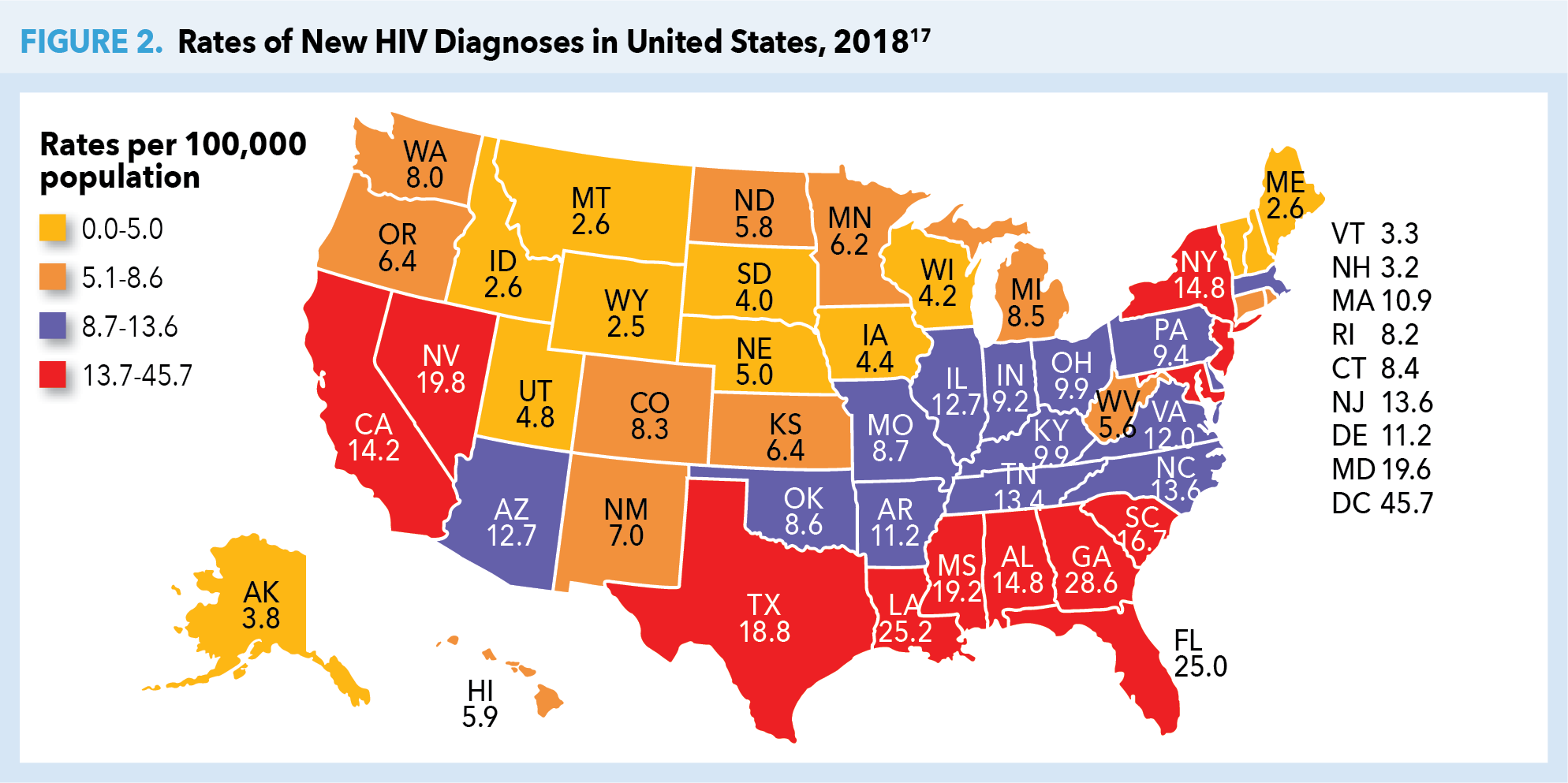
Perceived stigma, distrust of the medical system, lack of PrEP awareness among patients and clinicians, and limited access due to cost are theorized as potential reasons why PrEP uptake is currently low. Distrust of the medical system may be tied to stigma, as patients are often reluctant to speak with their health care providers about their sexual activity or behaviors of increased risk. This is especially true among MSM.18
Further, clinicians may be unfamiliar with how to monitor patients on PrEP, and may not realize that a less costly generic PrEP formulation is now available.19 In many locales, required preauthorization has been one of the most common barriers to prescribing PrEP, according to primary care and HIV-treatment providers.13 Without adequate insurance coverage, the annual cost of continuous PrEP use (including guideline-based interval visits, laboratory testing, and prescription refills) is more than $10,000. Although there are federal and state-level programs to increase access to PrEP, (see page 22, Your Patient’s Advocate: Coordination of Care and Access to Financial-Assistance Resources) cost may be a barrier for young black and Latino MSM and transgender women—many of whom are likely to be uninsured or underinsured—and who may benefit the most from PrEP.15
Building Upon an Established Practice Foundation
Through expansion of scopes of practice, pharmacists are demonstrating their ability to work in chronic disease management and to dispense or administer certain drugs and biologic products.1,14 Many states, in an effort to curb the soaring rates of drug overdose, now allow pharmacists to dispense naloxone without a prescription.15,20 Frequently, pharmacists in patient-centered medical homes lead medication-management services; help patients meet clinical-management goals as diverse as control of glycated hemoglobin (HbA1c), systolic blood pressure, and serum lipids; and increase vaccination rates and medication adherence.21 In certain states, including Colorado, upon meeting additional education, credentialing, or board recognition, pharmacists are authorized to independently prescribe or furnish and dispense contraceptives, tobacco cessation therapy, and travel medications.1,13 Further, community pharmacists are extremely well-positioned to address certain public health problems, since high-risk patients visit their community pharmacies more often than their primary care providers (PCPs).21
Within the realm of HIV disease management, pharmacists have already demonstrated high levels of success in increasing ART adherence through comprehensive means: identification of patient-specific barriers (eg, health literacy, cognitive factors, mental-health and substance-use comorbidities, economic factors); detection of therapy-related barriers (eg, adverse effects, drug-drug interactions); and recognition of service-delivery barriers (eg, patient-provider relationships, providers’ cultural competencies).22
An obvious next step in expanding the pharmacist’s role in HIV care is the prescribing and management of PrEP and PEP. Experienced in the principles and practices of chronic-disease medication management, and knowledgeable of HIV pharmacology, the pharmacist can play an essential role in HIV prevention across diverse practice venues.23-25
The Evolving Scope of Pharmacy Practice: The Future of HIV Prevention Is Here25
Despite Colorado’s relatively low HIV incidence—an estimated 470 new cases in 2019 per fourth-quarter estimates of the Colorado Department of Public Health and Environment (CDPHE)—there is an upward trend. The 2019 estimate reflects an approximate 13% increase over cases reported in 2018.26 This contrasts with overall US HIV incidence rates, which remained stable or showed slight decreases between 2014 and 2018.27
Responding to this public health imperative, to reduce HIV incidence in Colorado, Senator Alex Valdez and Representative Leslie Herod, along with 45 bipartisan State Senate and House cosponsors drafted and introduced legislation to expand PrEP and PEP access through inclusion of pharmacists as providers of HIV-prevention care. In June 2020, Colorado’s House Bill (HB) 20-1061 passed both the State Senate and House with striking majorities (30–3 and 47–17, respectively). HB 20-1061 Section 4 amends the Colorado Revised Statutes 12-280-103 to include prescribing and dispensing of preexposure and postexposure (nonoccupational) prophylaxis for the prevention of HIV acquisition and ordering of lab tests in conjunction with prescribing or dispensing the drugs (Table 1).1,28

Moreover, HB 20-1061 Section 2 mandates coverage of the pharmacy-based clinical or administrative service, either through direct medical billing or enhanced dispensing fees, at the same level the payer would cover these services if performed by a physician or advanced practice nurse. Section 3 restricts payers from requiring prior authorization or step-therapy for clients accessing HIV-prevention services at the pharmacy level. As these mandates are Colorado-specific, they do not apply to large, nationwide employer-based health plans or Medicaid- or Medicare-backed plans.1
Basic-Science Essentials for the Pharmacist
Therapeutic Interventions in the HIV Viral Life Cycle
HIV targets and replicates within CD4 T-lymphocytes (T helper cells)—cells that are critical to adaptive immune responses—leading to CD4 destruction and, thereby, decreasing the functionality of the immune system and increasing the risk of opportunistic infections.29,30
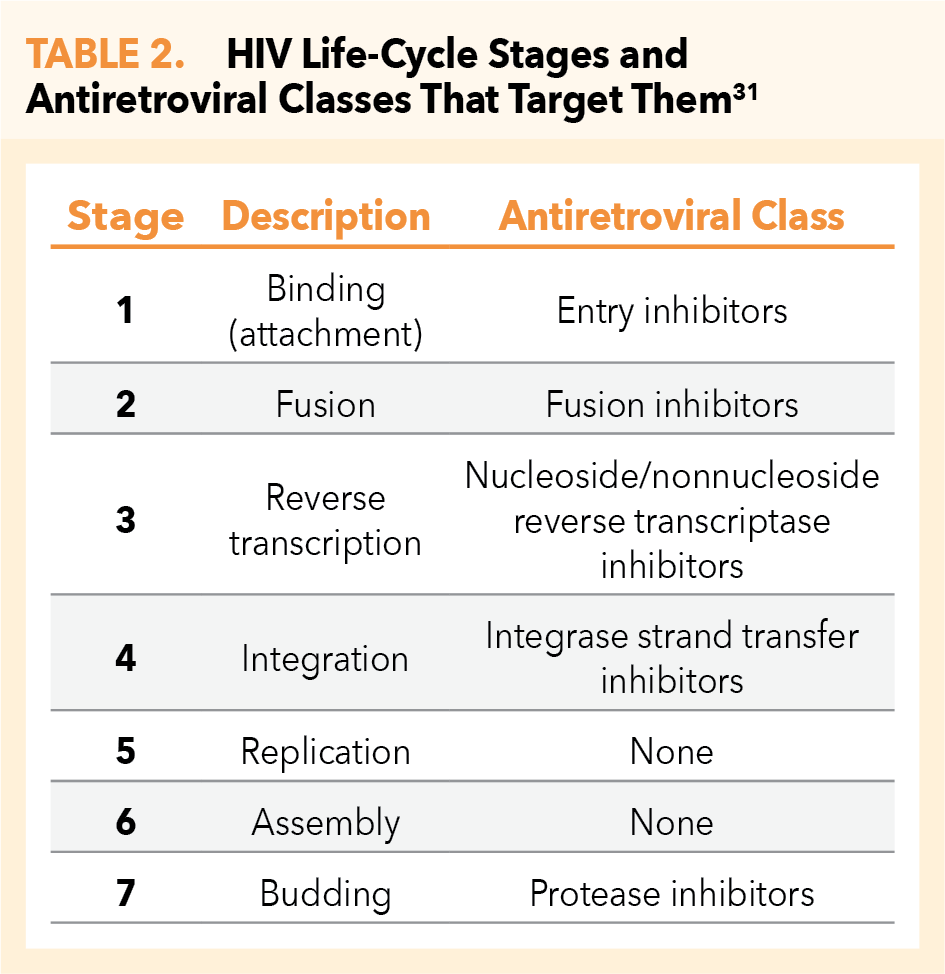
The HIV life cycle (Figure 3) consists of 7 stages, 5 of which inform the mechanisms of action of current ARTs (Table 2).31 The first stage is binding (or attachment): HIV binds to receptors on the surface of a CD4 cell. The second stage is called fusion: upon attachment, the viral envelope fuses with the CD4 cell membrane, allowing HIV to enter the host cell and release viral components. Stage 3 is reverse transcription: inside the CD4 cell, HIV releases and uses a reverse transcriptase enzyme to convert HIV RNA (ribonucleic acid) into HIV DNA (deoxyribonucleic acid). HIV DNA then enters the nucleus and proceeds to integration (stage 4), during which HIV releases integrase, an enzyme that integrates viral DNA into the DNA of the host CD4 cell. In stage 5, replication, the machinery of the host CD4 cell produces long chains of HIV proteins (building blocks for more HIV); while in stage 6, assembly, new HIV proteins and HIV RNA move to the surface of the cell and combine as immature (noninfectious) HIV. The seventh and final step in the HIV life cycle is budding, wherein newly formed immature HIV pushes itself out of the host CD4 cell. Protease, an HIV enzyme released from the new HIV breaks up long protein chains in the immature virus, creating a new and mature (infectious) virus.31
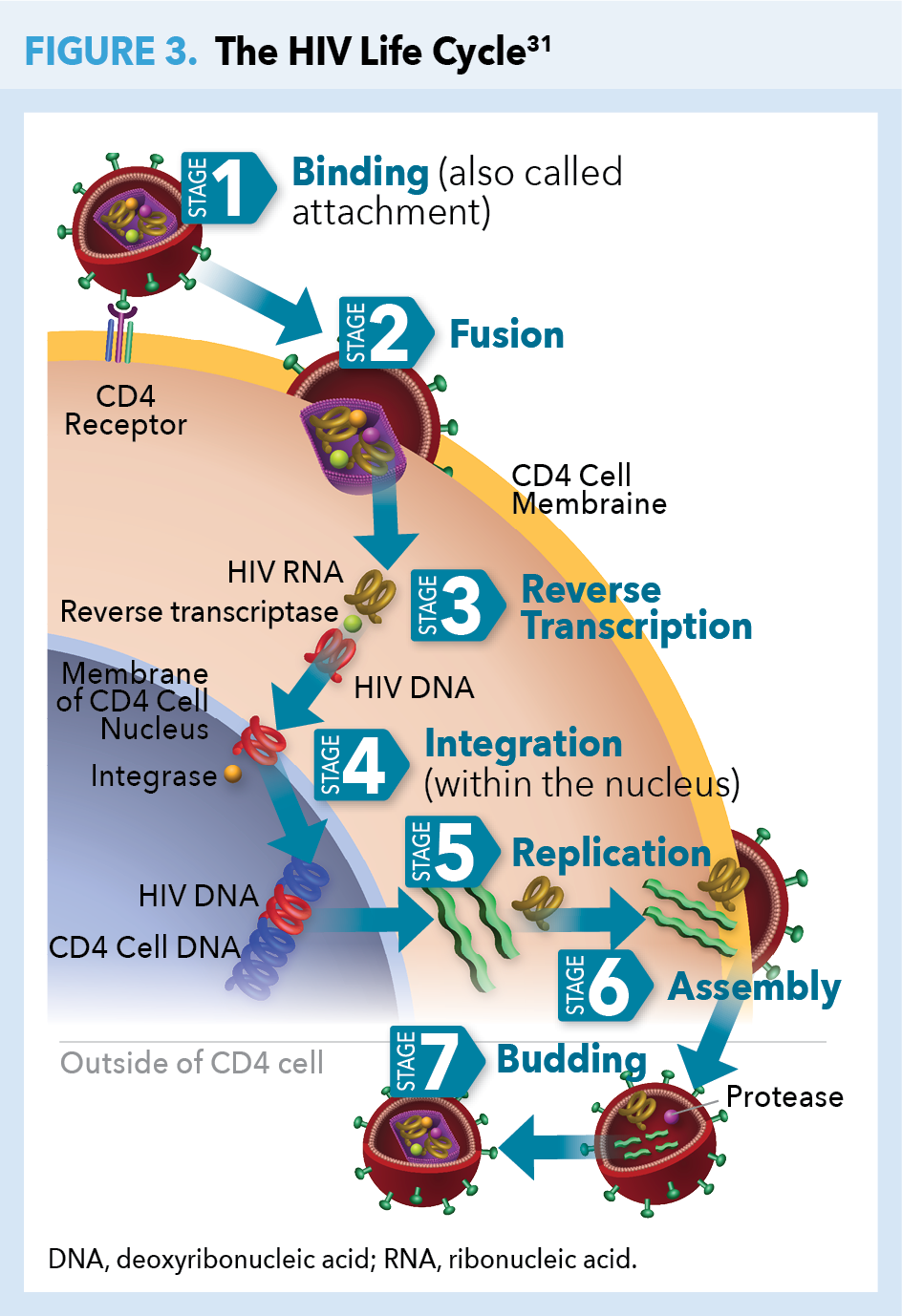
Understanding the Immunologic Basis for HIV Testing
Following release of mature HIV from the CD4 cell, the viral RNA is initially present in the plasma without recognition by the host immune system. By approximately day 17, the HIV p24 antigen is present. The body recognizes this viral protein as foreign, thereby eliciting an immune response with production of specific anti-HIV antibodies. During this initial 17-day period neither HIV antigen (Ag) nor anti-HIV antibodies (Ab) are detectable with HIV tests used most commonly in the community setting. Yet HIV RNA may be detectable as early as day 10 with more highly specialized nucleic acid amplification testing (NAAT). That is, during this window period, an HIV-infected person may have an HIV-negative test result (Figure 4).32,33
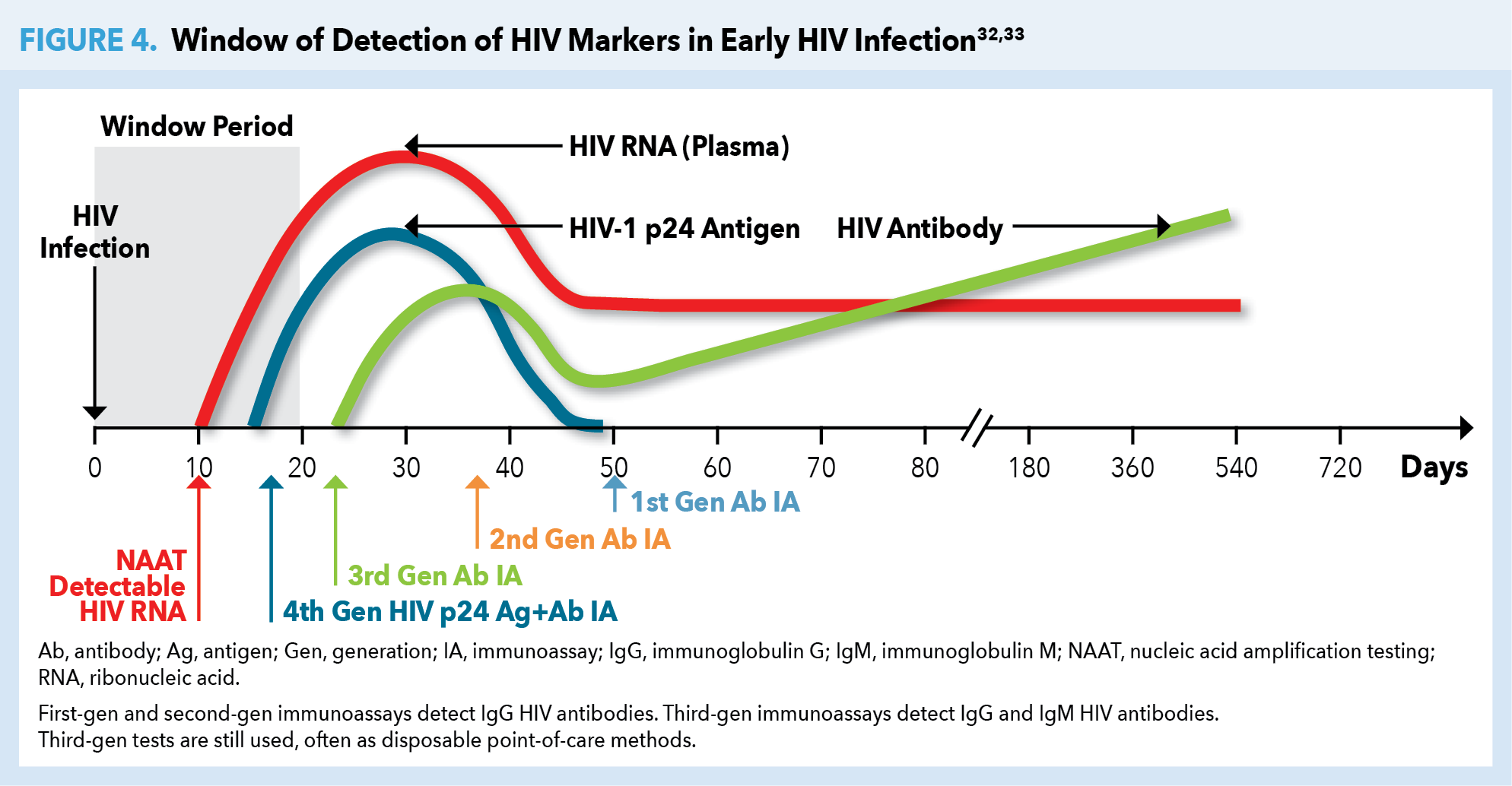
Use of 4th-generation (gen) antigen/antibody (Ag/Ab) tests is the current standard of care. Usually performed in the laboratory setting, this immunoassay (IA) detects both p24 antigen and HIV-specific antibodies at approximately day 18. Third-gen IAs detect immunoglobulin G (IgG) and immunoglobulin M (IgM) HIV antibodies at about day 23. Third-gen tests are still used, often as a point-of-care testing modality. A negative HIV test following a high-risk exposure should always be clinically correlated with any signs or symptoms of acute HIV infection, and repeat testing after the window period should be advised.32,33
PrEP 101
What Is PrEP?
PrEP is the use of specific combinations of antiretroviral agents to reduce the risk of HIV infection. It is intended for HIV-negative individuals who are at risk of infection through sexual intercourse or injection drug use, including sexually active MSM, heterosexual men and women, and transgender persons. PrEP is also an alternate prevention strategy for individuals receiving PEP who continue to engage in high-risk behavior or who have received multiple courses of PEP. Sexually active adults and adolescents are defined as people engaging in anal or vaginal sex in the past 6 months.34
At the time of this publication, CDC guidelines recognize only 2 drug regimens for use as PrEP.34,35
- Tenofovir disoproxil fumarate (TDF) 300 mg/emtricitabine (FTC) 200 mg (fixed-dose combination) once daily (Truvada®, US Food and Drug Administration [FDA]-approved for treatment of chronic HIV infection in 2004 and FDA-approved for PrEP in 2012)36
- Approved for all persons at risk of HIV acquisition
- Currently the only PrEP regimen with an FDA-approved generic formulation (available as of October 2020) 19
- Tenofovir alafenamide (TAF) 25 mg/FTC 200 mg (fixed-dose combination) once daily (Descovy®,
FDA-approved for PrEP, October 2019)37
- Indicated for all persons at risk of HIV acquisition, with the current exception (ie, has not been studied) of those at risk through receptive vaginal sex or injection drug use
PrEP Pharmacologic Considerations: Efficacy and Safety
Key clinical trials involving TDF/FTC used as PrEP—including iPrEx (Iniciativa Profilaxis Pre-Exposición [Preexposure Prophylaxis Initiative]), Partners PrEP (Partners Pre-Exposure Prophylaxis Study), FEM-PrEP (Preexposure Prophylaxis Trial for HIV Prevention Among African Women), and TDF2 (Botswana TDF/FTC Oral HIV Prophylaxis trial)—demonstrated safety and efficacy of the combined use of these 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) as PrEP across all at-risk populations, including MSM, heterosexual HIV-discordant couples, heterosexual men and women, and people who inject drugs (PWID).34,38-42 As noted, TDF and FTC target reverse transcription, stage 3 in the HIV life cycle (Figure 3). Once phosphorylated intracellularly, creating diphosphate and triphosphate metabolites, respectively, these nucleotide/nucleoside analogs inhibit HIV reverse transcriptase by competing with viral substrates and, thereby, causing DNA chain termination and preventing viral RNA conversion.36
Whereas older NRTIs were characterized by serious toxicities (including bone marrow suppression, peripheral neuropathy, lactic acidosis, and pancreatitis) secondary to their effects on human cellular mitochondrial DNA, these newer NRTIs are weaker inhibitors of mitochondrial DNA and are less likely to result in significant toxicities.43
TDF/FTC remained the sole FDA-approved antiretroviral regimen for use as PrEP until October 2019. The randomized, double-blind, active-control DISCOVER trial evaluated the efficacy and safety of TAF/FTC vs TDF/FTC in high-risk cisgender MSM and transgender women (in North America and Europe).44 Eligible patients (N=5387; mean age 36 years) had engaged in ≥2 episodes of condomless anal sex in the preceding 12 weeks or received the diagnosis of rectal gonorrhea/chlamydia or syphilis in the preceding 24 weeks. The primary endpoint was the rate of HIV infection per 100 person-years (PY) when 50% of patients had completed 96 weeks of PrEP use. Longer term results, reported once all participants completed 96 weeks, showed that just 23 HIV diagnoses occurred during the study period—an infection rate of 0.16/100 PY for TAF/FTC vs 0.30/100 PY for TDF/FTC (Figure 5).45
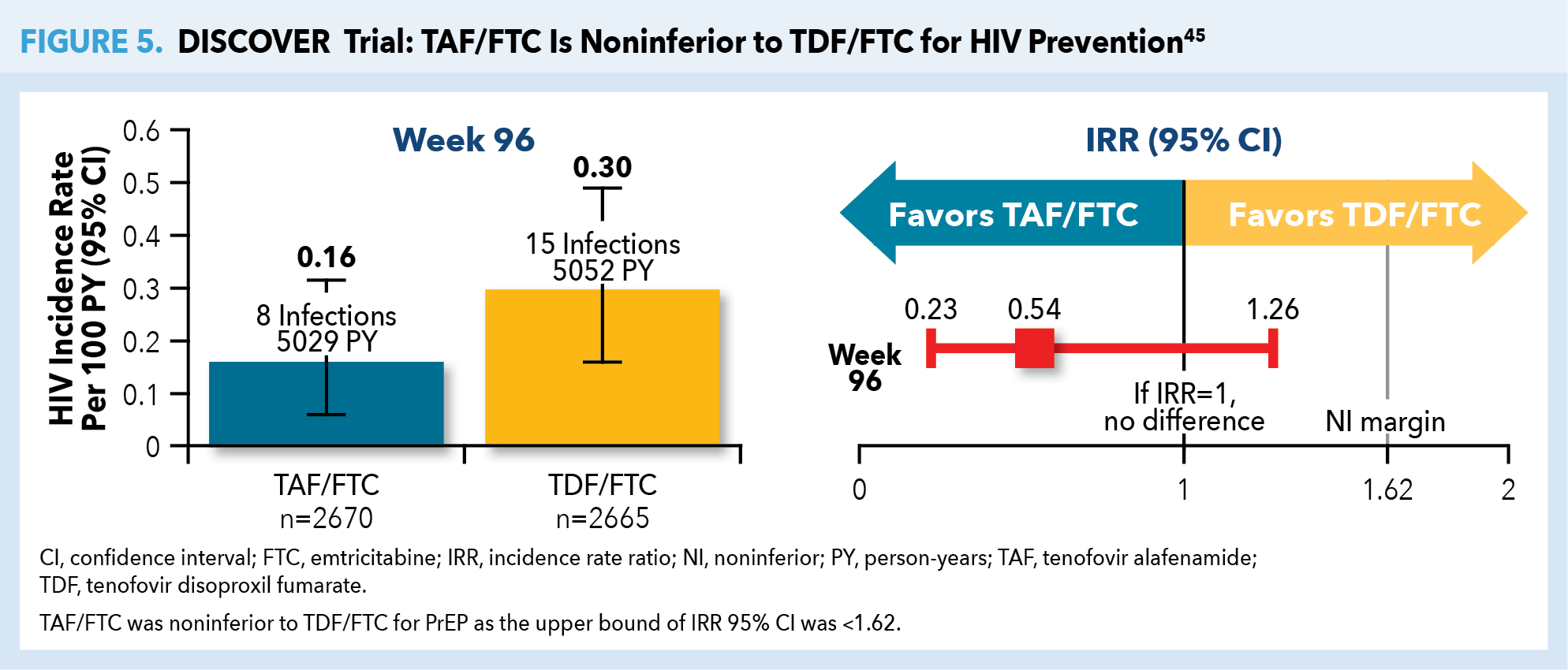
TAF/FTC was associated with superior bone and renal safety profiles compared with TDF/FTC, although both combinations were well-tolerated and associated with low rates of discontinuation due to adverse events. Combination TAF/FTC was considered noninferior to TDF/FTC in preventing HIV infection, thereby leading to expanded PrEP medication options for persons at risk of HIV acquisition, except for those at risk through receptive vaginal sex or injection drug use.37,45
Both TDF and FTC are primarily excreted through the kidneys; therefore, patients with impaired renal function—based on estimated creatinine clearance (eCrCl), as calculated using the Cockcroft-Gault equation—are at risk.46 Use of TDF/FTC as PrEP is not recommended for persons with eCrCl <60 mL/min/1.73 m2. Further, reduced-dosing strategies for TDF/FTC as PrEP have not been developed.34
In contrast, TAF is metabolized by the lysosomal multifunctional enzyme cathepsin A and can be used in patients with more compromised renal function (eCrCl ≥15 mL/min/1.73 m2) or patients on hemodialysis. Note, however: the FTC component of TAF/FTC is not recommended for patients with eCrCl <30 mL/min/1.73 m2 and, therefore, TAF/FTC for PrEP is not recommended for patients with eCrCl <30 mL/min/1.73 m2).37 The serum half-life of TAF is 0.5 hours, with an intracellular half-life of 150–180 hours. In contrast, the serum half-life of TDF is 17 hours, with an intracellular half-life of >60 hours. The bone- and renal-related adverse effects associated with TDF are less likely to occur with TAF due to this lower plasma exposure.47 Of note, in the context of HIV treatment (ie, not PrEP), switching patients who show a decline in bone mineral density from TDF-containing to alternative ART regimens was shown to increase bone mineral density, but the clinical significance of this increase remains unknown.48
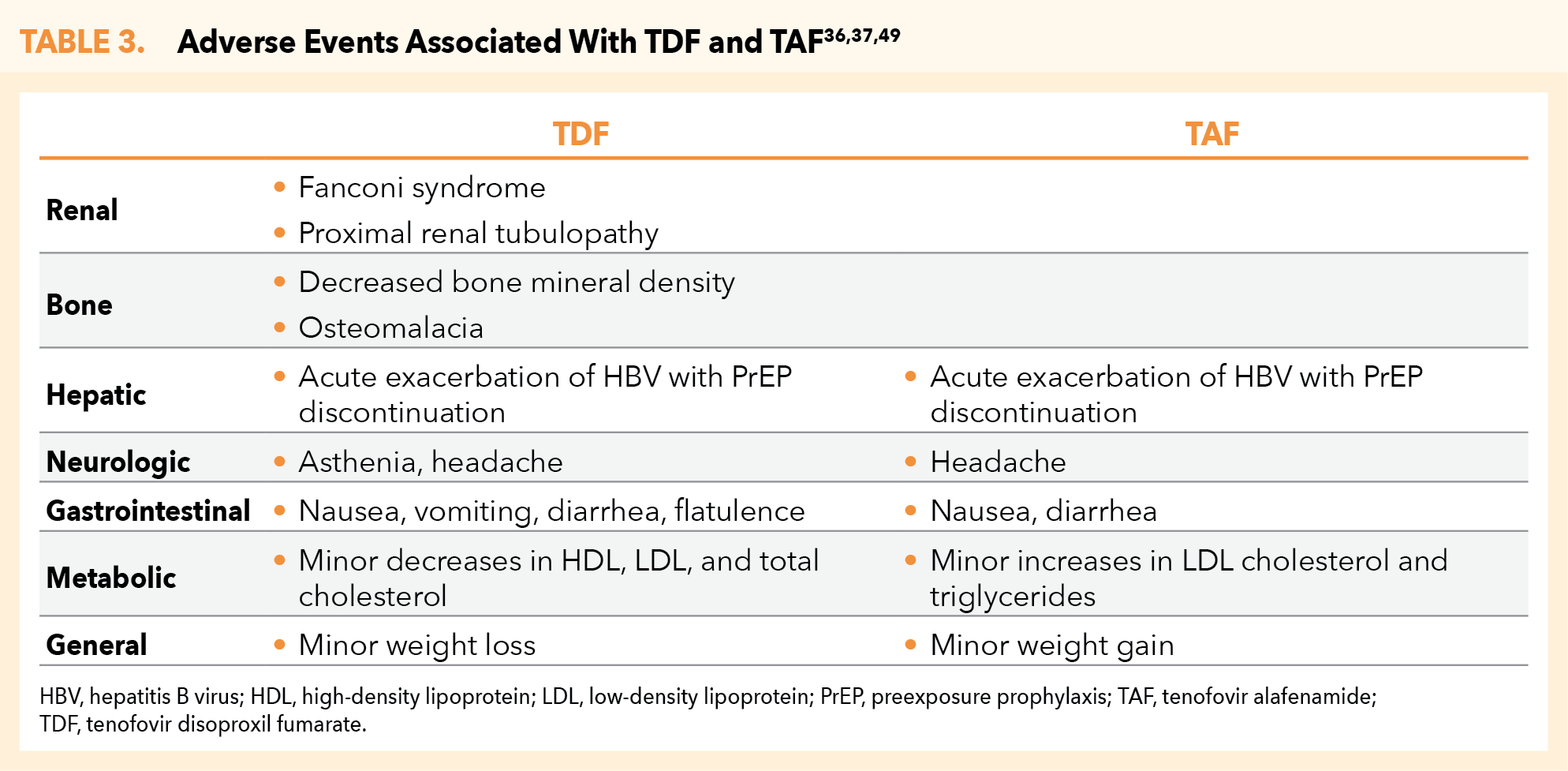
While TAF/FTC appears to have safer kidney and bone toxicity profiles than TDF/FTC, TDF/FTC is associated with lower lipid levels and weight loss.34,36,37,49 However, the CDC PrEP guidelines do not provide further parameters for consideration when choosing between these 2 PrEP regimens.35 Other, less significant or transient adverse effects of TDF/FTC include asthenia, headache, nausea, vomiting, diarrhea, flatulence, minor decreases in high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol, and minor weight loss.36 TAF/FTC may be associated with headache, nausea, diarrhea, minor increases in LDL cholesterol and triglycerides, and minor weight gain (Table 3).37
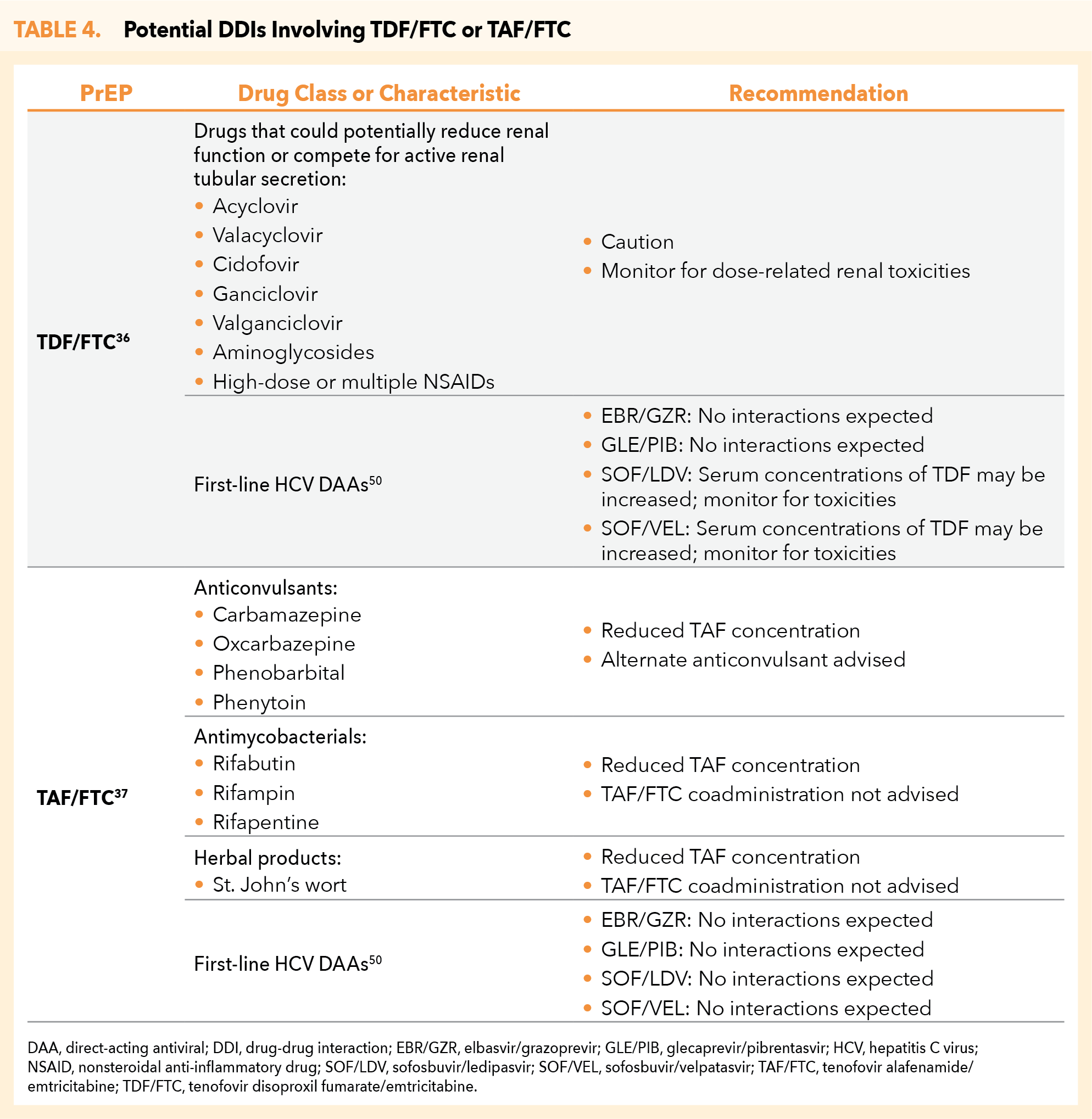
Potential drug-drug interactions (DDIs) with TDF/FTC or TAF/FTC are listed in Table 4.36,37,50 As the incidence of hepatitis C virus (HCV) is increased in populations at risk of HIV acquisition, first-line HCV direct-acting antiviral (DAA) regimens are listed, including those with no potential DDIs.
The start-up syndrome of headache, nausea, and flatulence occurring in the first month of TDF/FTC use as PrEP occurs in a minority of patients and typically resolves within 3 months, sooner for many patients. Patients should be forewarned of this possibility, instructed on the use of over-the-counter medications for symptom management, and educated regarding more serious signs and symptoms—including those of acute HIV infection—that should be reported on an urgent basis.31,51
Pretreatment Determination of PrEP Eligibility2,34
Determination of an individual’s eligibility for PrEP includes several required clinical components:
- Conduct a comprehensive behavioral risk assessment (sexual; injection drug use) to identify appropriate PrEP candidates
- Document evidence of HIV-negative status with use of an FDA-approved blood test, or CLIA-waived rapid point-of-care fingerstick blood test performed within 7 days of therapy initiation (CLIA-waived indicates that under the Clinical Laboratory Improved Amendments, a laboratory test can be conducted without the need for more stringent standards)
- Note: Per SBOP Rule 17, oral swab tests and patient reports are not acceptable as evidence of negative HIV status
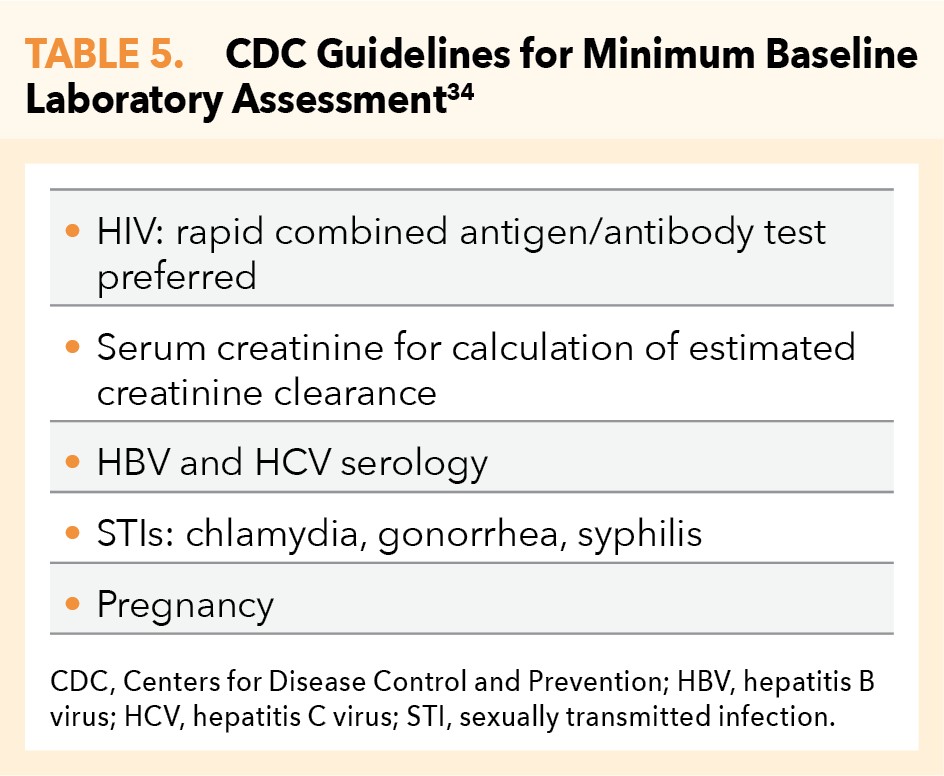
- Perform baseline (pretreatment) laboratory assessment (Table 5) and clinically correlate results
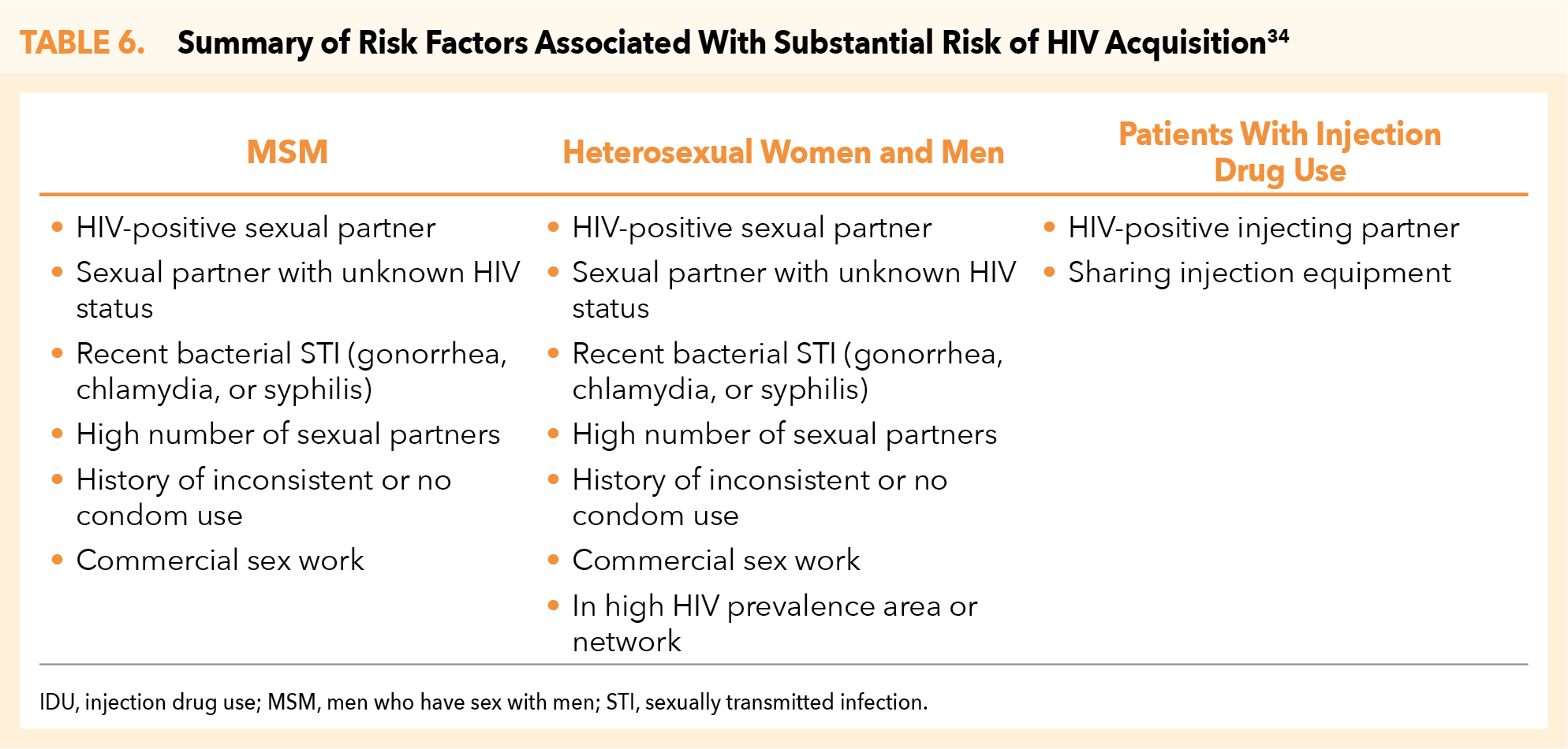
Comprehensive Behavioral Risk Assessment (Sexual and Injection Drug Use)34
A comprehensive behavioral risk assessment (including sexual and injection drug use) is critical for identification of appropriate PrEP candidates (Table 6).
The behavioral assessment may also reveal additional needs for in-depth education, risk-reduction counseling, and community-based patient-support services. Embracing Sexual Wellness and Discussing HIV Risk: The Sexual Health Assessment (page 20) provides a more in-depth discussion of these concerns.
Confirmation of HIV-Negative Status
Currently, 4th-gen combined antigen/antibody tests are both recommended and preferred. Antigen/antibody tests utilizing blood from a vein can usually detect HIV infection 18–45 days after an exposure, while rapid antigen/antibody tests with fingerprick blood may detect HIV 18–90 days after exposure (Figure 4).32,33,52 Other HIV tests are recognized and allowed, but it is crucial to remember the limitations of antibody-only tests. As of June 2018, CDC-approved, CLIA-waived rapid HIV tests, suitable for use in nonclinical settings, and involving fingerstick or venous whole blood, include but are not limited to the following
- Chembio SURE CHECK® HIV 1/2 Assay
- Clearview® HIV 1/2 STAT-PAK®
- Alere Determine™ HIV-1/2 Antigen/Antibody Combined Test
- INSTI® HIV-1/HIV-2 Antibody Test
- Uni-Gold™ Recombigen® HIV-1/2 Antibody Test

Only the Alere Determine™ test is a 4th-gen combined antigen/antibody assay; all others listed test only for antibodies to HIV-1 and HIV-2.53
The following may be performed using oral-fluid swab (or fingerstick or venous whole blood). Note, however, oral-fluid swabbing should not be used to document HIV status.
- Chembio DPP® HIV-1/2 Assay (oral test; not acceptable for confirmation of HIV status)
- OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test53
Rapid tests using finger-prick or venous whole blood can yield results in approximately 10 minutes. No further testing is needed if test results are negative, but positive tests must be confirmed with more specific conventional blood tests, with results available in <1 hour to several days. Oral HIV tests are not recommended in these clinical scenarios.31 The CDC provides a website listing FDA-approved HIV diagnostic tests including a list of CLIA-waived tests: https://www.cdc.gov/hiv/testing/laboratorytests.html.
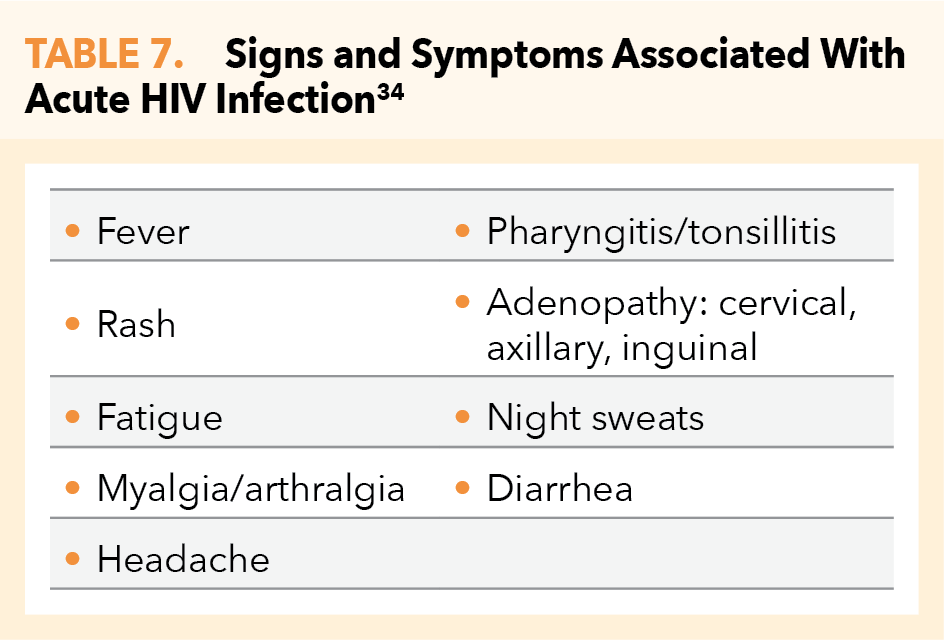
When interpreting HIV-test results, clinicians must keep in mind the approximate 18-day period (see Figure 4), during which time even the 4th-gen combined antigen/antibody tests may fail to detect early infection. Therefore, all patients should be questioned regarding specific signs and symptoms suggestive of or consistent with early/acute HIV (Table 7). Only with both negative HIV testing and negative screening for these signs and symptoms should there be further consideration of PrEP prescribing.34
Laboratory Assessment for PrEP-Associated Risks
Estimated creatinine clearance (as calculated using the Cockcroft-Gault equation) is critical to determination of PrEP eligibility.
- If eCrCl <60 mL/min/1.73 m2, TDF/FTC is not recommended; reduced-dosing strategies for TDF/FTC as PrEP have not been developed34
- If eCrCl <30 mL/min/1.73 m2, TAF/FTC is not recommended37
Pretreatment evaluation of hepatis B (HBV) serology is equally important. While HBV infection is not a contraindication to use of PrEP, HBV status must be known and documented prior to PrEP initiation, as discontinuation of PrEP may be associated with acute exacerbation of HBV. Patients positive for hepatitis B surface antigen (HBsAg) should be referred to an appropriate clinician for evaluation and consideration for HBV treatment.34
TDF/FTC is a safe and effective HIV-prevention strategy for women who are at substantial risk of HIV acquisition including during conception, pregnancy, and the postpartum period. Note: TAF/FTC is not approved in those having receptive vaginal sex. Continuing TDF/FTC during breastfeeding may be beneficial for some women; however, the long-term safety of infant exposure to TDF/FTC has not been determined. TDF/FTC does not reduce the effectiveness of oral contraceptives.34
Patient-Centered PrEP: Regimen Selection and Pretreatment Counseling
Once the patient’s eligibility is established, few considerations will impact the selection of PrEP regimen. The most critical is the patient’s renal function: TDF/FTC should not be prescribed for individuals whose eCrCl is <60 mL/min/1.73 m2, because of TDF’s renal clearance.34,37 Bone density (especially if the patient is over 50 years of age), cholesterol, and weight change may also be considerations for the given individual.34,37 Mode of sexual exposure may influence PrEP regimen selection: as mentioned previously, TAF has not been approved for those at risk through receptive vaginal sex.35 Further, the patient’s potential for drug-drug interactions should be assessed based on all currently taken medications. In real-world clinical practice, selection of PrEP regimen may come down to the practical concerns of insurance coverage and cost.15 A generic formulation for TDF/FTC became available as of October 2020, but TAF/FTC is available only in brand form.19
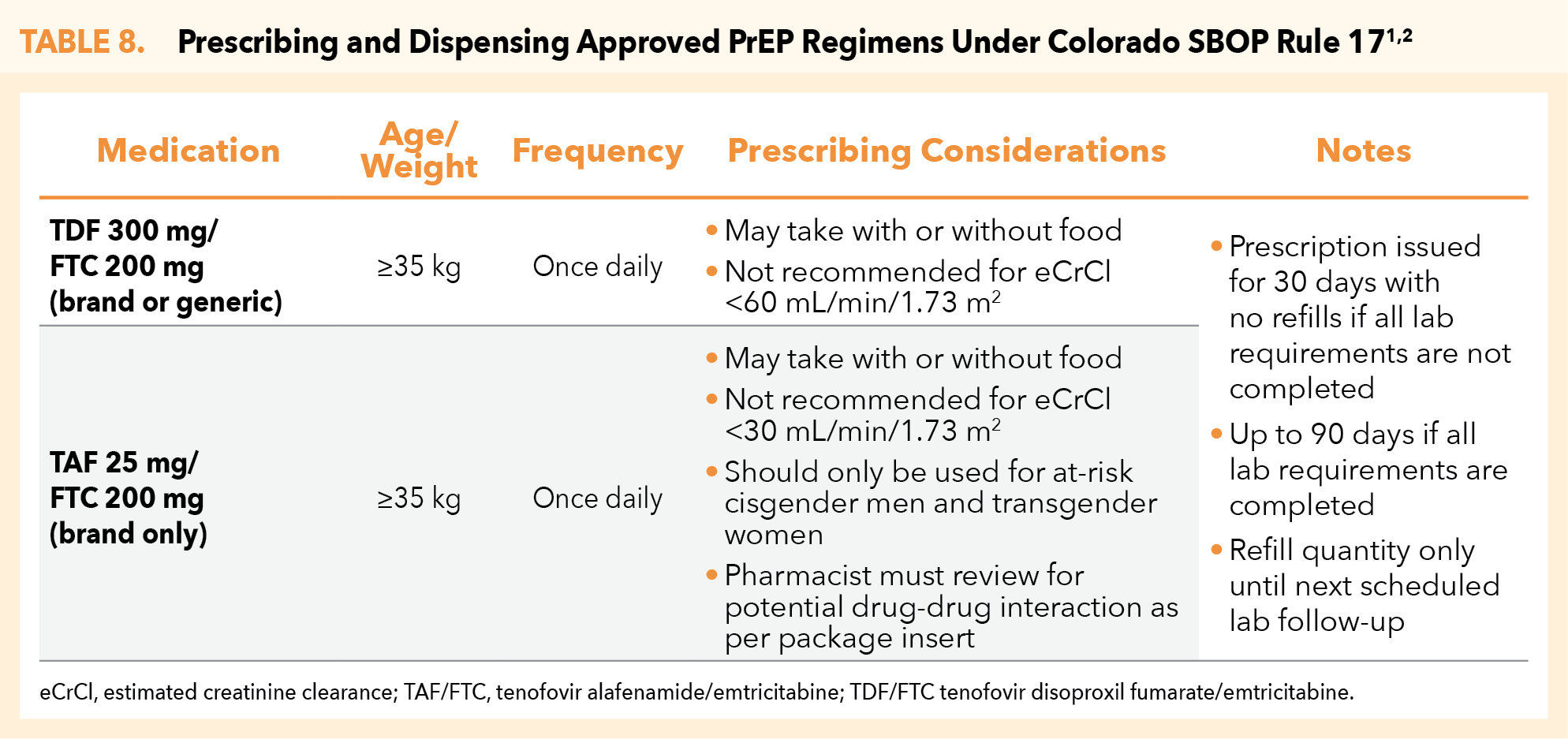
Under CO HB 20-1061 and CO SBOP Rule 17, the pharmacist may prescribe and dispense the following FDA-approved PrEP regimens (Table 8).1,2
Having established the patient’s PrEP eligibility, the PrEP provider’s additional pretreatment requirements address the following clinical activities34:
- Prescribe safe and effective PrEP regimens for eligible HIV-uninfected persons
- Educate patients about the selected PrEP regimen to maximize safe use
- Provide counseling and effective contraception to women on PrEP who do not wish to become pregnant
- Provide other patient-centered counseling regarding the following:
- Medication adherence to achieve and maintain protective tissue levels
- HIV risk reduction; including discussion/provision of prevention-services referrals
- Review PrEP discontinuation and resumption requirements
Emerging (nonapproved) PrEP Regimens
While Table 8 presents TDF 300 mg/FTC 200 mg and TAF 25 mg/FTC 200 mg as the only currently approved PrEP regimens, SBOP Rule 17 states, “Other FDA-approved/CDC-recommended medication or regimens can be used if they become available.”1,2
Of particular note, preliminary results of the HIV Prevention Trials Network 083 (HPTN 083) study of the efficacy of the integrase inhibitor cabotegravir (CAB) as a long-acting injectable for HIV prevention were presented at the AIDS 2020: Virtual conference held July 6–10, 2020. This National Institutes of Health (NIH)-funded multisite study of 4566 cisgender MSM and trans women who have sex with men randomized participants to receive CAB as a long-acting injectable every 8 weeks or daily oral TDF/FTC. HPTN 083 was terminated in early 2020 after the CAB group was shown to have statistically superior outcomes when compared with the TDF/FTC group, with the CAB group showing 66% lower risk of HIV infection. It should be noted that both study groups received highly effective HIV-prevention regimens: incidence rates of HIV infection were 0.41% in the cabotegravir group, and 1.22% in the TDF/FTC group (Figure 6).54 Approval of CAB for use as PrEP by MSM and trans women who have sex with men is anticipated in the near future.
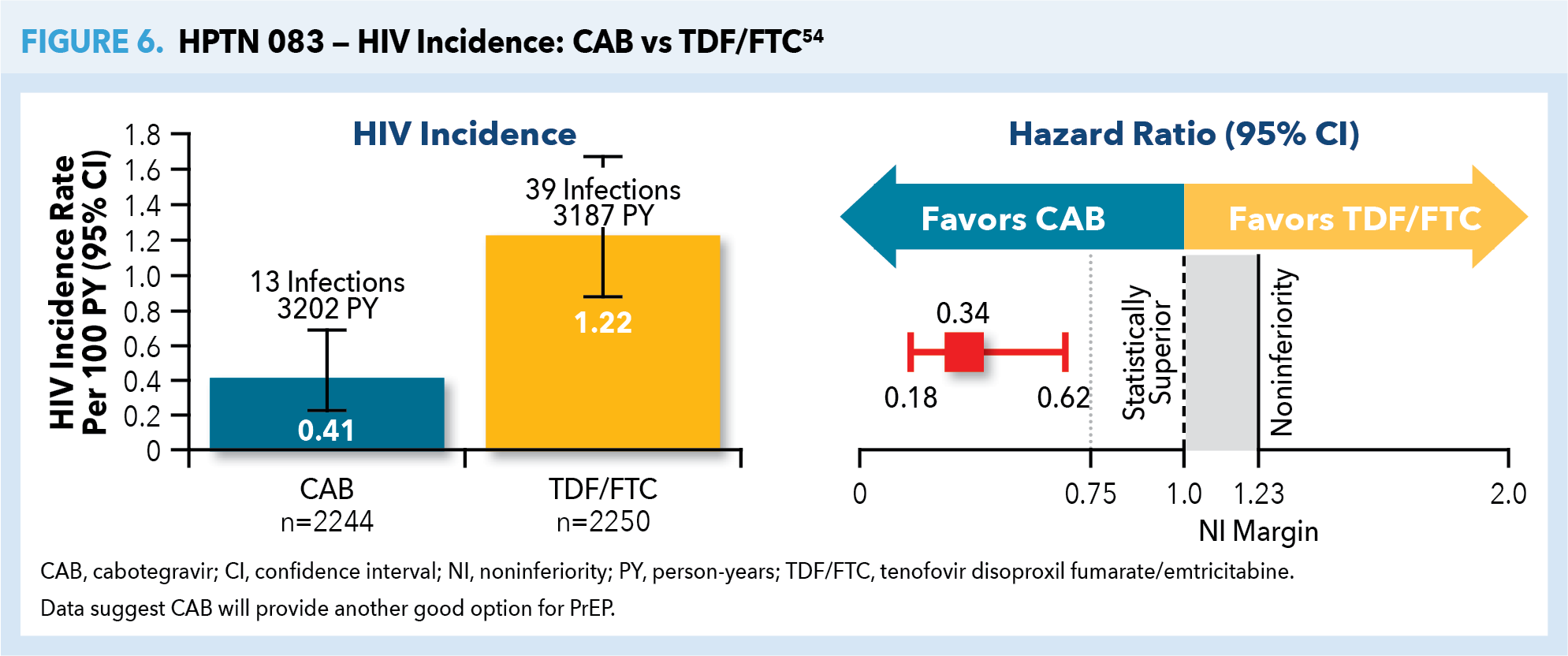
The HIV Prevention Trials Network is currently studying the effectiveness of long-acting CAB as PrEP in HIV-uninfected women. HPTN 084 (LIFE) is a phase 3 trial designed to track roughly 3200 women of childbearing age in sub-Saharan Africa over a 4.5-year period. While both methods were highly effective—HIV incidence of 0.21% (95% CI 0.06%–0.54%) in the CAB group and 1.79% (95% CI 1.24%–2.51%) in the TDF/FTC group—long-acting cabotegravir was 89% (95% CI 68%-96%) more effective than TDF/FTC. The study was terminated early (November 2020) because of this demonstration of CAB’s statistically based superiority.55 Similarly, approval for CAB for use as PrEP in women of childbearing age is anticipated. This long-acting, injectable PrEP option has the potential advantage of enhanced HIV prevention without reliance on daily adherence to an oral PrEP regimen for women, as well as MSM and trans women who have sex with men.
Alternative (nonapproved) PrEP Strategies
Note: Colorado SBPO Rule 17 does not authorize the following 2 PrEP strategies.
CDC guidelines provide for use of TDF monotherapy for PWID and for heterosexual men and women, based on prior trials demonstrating efficacy in these specific populations. There are no data supporting TDF monotherapy for MSM and, therefore, it is not recommended.34
While not CDC-recommended or FDA-approved, evidence-based on-demand or event-driven protocols are endorsed by other organizations. For example, 2-1-1 is a PrEP dosing protocol specifically recommended for MSM engaging in anal sex without condoms (not for vaginal sex) by the International Antiviral Society–USA (IAS–USA) and the World Health Organization (WHO).56,57 Named on the basis of the number and timing of PrEP tablets taken, 2-1-1 provides the following recommendations58:
- A loading dose of 2 TDF/FTC tablets is taken 2–24 hours before anal sex
- A third tablet is taken 24 hours after the first dose
- A fourth tablet is taken 24 hours later
- If there is another sexual exposure within 7 days of the last dose, 1 tablet is taken 2–24 hours before anal sex, 1 tablet 24 hours after the first dose, then 1 tablet 24 hours after the second dose
The efficacy of this dosing protocol was demonstrated in the IPERGAY study, the scope of which was limited to TDF/FTC. The preceding 2-1-1 recommendations do not apply to TAF/FTC.56,58
On-PrEP Interval Laboratory Testing and Patient Monitoring
The following table provides a comprehensive look at CDC guidelines for on-PrEP interval laboratory testing and patient monitoring (Table 9).34
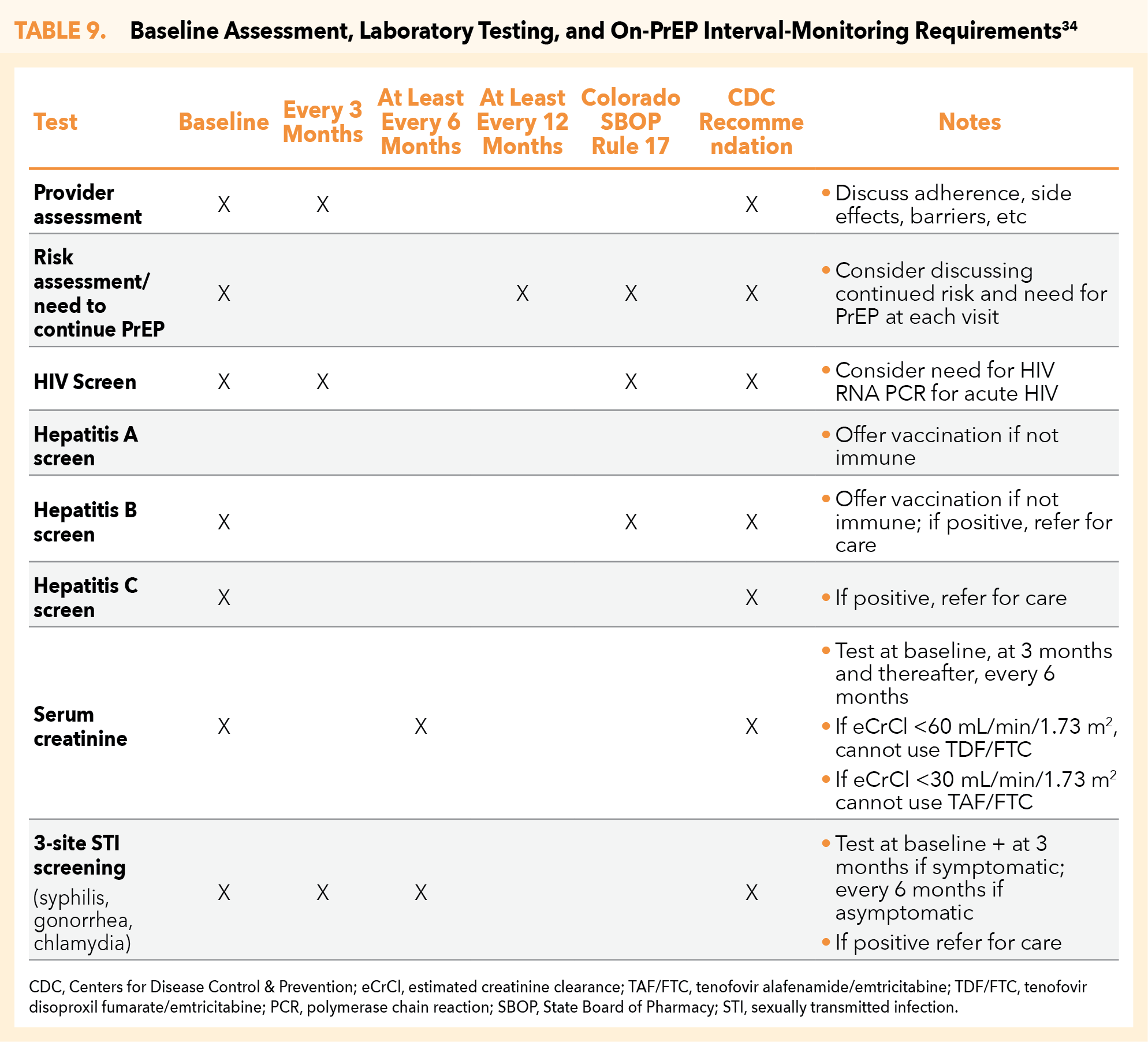
CO SBOP Rule 17 allows for the pharmacist’s continuous prescribing of approved PrEP therapies, as based on adherence to monitoring requirements. An initial 30-day supply of the selected PrEP medication can be prescribed and dispensed by the pharmacist following confirmation of the patient’s HIV-negative status. The pharmacist may authorize PrEP refills beyond the initial 30-day prescription as long as laboratory and monitoring requirements are fulfilled.2
Indications for Referral
If at any point in the PrEP care continuum—from baseline evaluation to long-term monitoring—acute HIV is suspected, a patient tests positive for HBV, becomes pregnant, has symptoms or laboratory findings of acute renal injury, or has a positive STI screen, referral to a PCP or appropriate provider should be made as soon as possible (Table 10).2

Patient Education, Counseling, and Support for PrEP Adherence
Components of basic medication-related education for patients include the following:
- Which PrEP medication is being prescribed? How is it taken? What is the dosing schedule? (Table 8)
- Time to maximum HIV-prevention effectiveness, as based on exposure mode (Table 11)
- How to manage missed doses
- Common signs and symptoms suggestive of acute HIV infection (Table 7)
- Common side effects of the prescribed PrEP regimen (Table 3)
- What quantity will be dispensed? (Table 8)
- Requirement to remain under the care of the PrEP prescriber for required interval monitoring
Time to maximum HIV prevention varies with mode of sexual exposure (Table 11). Patients should be advised that condom use is critical while tissue concentrations remain low, and that ongoing, consistent use of condoms is necessary for the prevention of both HIV and other STIs.34
Effective HIV prevention requires a high level of daily adherence to the PrEP medication, but there is some leeway for missed doses. The STRAND trial showed that 6 to 7 doses per week are required to maintain protective vaginal tissue concentration; fewer doses per week may be adequate to maintain colorectal tissue concentrations. Nonetheless, daily adherence should be stressed for all persons on PrEP. Should a patient report missing a dose, instruct the patient to take a single missed dose as soon as it is remembered. If, however, it is nearly time for the next dose, the patient should proceed with the regular dosing schedule.34
Supporting the Patient’s PrEP Adherence
Key tactics for successful medication-adherence counseling include the following:
- Simplified education and explanations; eg, dosage schedule, impact of adherence on PrEP efficacy
- Strategies that address patient-specific adherence barriers; eg, substance use
- Nonjudgmental adherence monitoring; eg, normalizing occasional missed doses, reinforcing success, encouraging condom use
PrEP adherence is supported by effective HIV risk-reduction education. Counseling should include information about substance use and protection from STIs with consistent condom use.34,59,60 Whether recreational and/or associated with injection practices, psychotropic substances may decrease inhibitions, increase risk tolerance, and reduce self-efficacy with regard to risk-reduction practices.61 Patients also should be reminded that PrEP does not protect against other STIs. Such personal risk-reduction practices—with emphasis on consistent condom use—remain critical, even when one is using PrEP. Adherence should also include:
- Acknowledgment of the effort required for behavior change
- Reinforcement of success
- Assistance with turning around any missteps along the way34
Additional adherence strategies can be found in the provider supplements to the CDC PrEP guidelines62; https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2017.pdf
As adverse effects may impact PrEP adherence, pharmacists should counsel patients regarding the following concerns34:
- Common side effects (headache, nausea, and flatulence) that typically resolve within the first month of taking PrEP (start-up syndrome) and over-the-counter medications for symptom management
- Signs or symptoms of acute HIV infection (Table 7) or acute renal injury, both of which require urgent intervention
PrEP Discontinuation and Resumption
Patients should receive specific counseling on how to safely discontinue and restart PrEP. Discontinuation may be prompted by the individual’s personal choice, changed life circumstances, nontolerated side effects, toxicities, chronic nonadherence, or acute HIV infection. The protection provided by PrEP wanes over the 7–10 days following discontinuation; therefore, alternative methods to reduce risk of HIV acquisition should be discussed, including the emergent use of PEP in the event of a high-risk exposure.34
Per CDC guidelines, PrEP discontinuation for any reason should be documented by the PrEP provider. This should include HIV status at the time of discontinuation, reason for discontinuation, recent PrEP adherence, and reported sexual risk behavior. Should the patient choose to resume PrEP at some point after discontinuation, all pretreatment requirements must be repeated and fulfilled. The PrEP provider should engage the patient in an open discussion regarding change in personal circumstances and commitment to PrEP adherence.34
Transitioning From PrEP to HIV Treatment as Prevention
Patients taking PrEP may become HIV-positive for various reasons. If detected at the first follow-up visit after PrEP initiation, it may be due to the patient having had undetectable acute infection at the time of pre-PrEP HIV testing (ie, within the window period). If detected at a later follow-up visit, this may indicate adherence issues.34 Rarely, some patients become HIV-positive despite good PrEP adherence: risk of HIV acquisition is not completely eliminated by high levels of adherence and may be due to drug-resistant viral strains.63
US Department of Health and Human Services (DHHS) HIV/AIDS guidelines recommend that upon laboratory confirmation of HIV infection in an individual on PrEP, the patient should transition from PrEP to comprehensive HIV treatment. An HIV treater (specialist or PCP) should be involved in determining ART selection and initiating therapy. Under new guidelines for rapid ART initiation (rapid ART; rapid start), there is no need to wait for additional laboratory results. A rapid ART strategy seeks to accelerate the time from HIV diagnosis to engagement in care and ART uptake and, ultimately, viral suppression. Current guidelines call for ART initiation on the day of diagnosis (or as close as possible).49 Patients should be counseled regarding the benefits of early/continuous viral suppression for both themselves and their partners, with emphasis on undetectable=untransmittable (U=U). That is, maintenance of viral suppression (an undetectable viral load on ART), helps prevent the transmission of HIV to others. This is the basis of HIV treatment as prevention.64
Summary of Colorado State Board of Pharmacy Statewide Protocols (Rule 17 Appendix C) Regarding PrEP
HB 20-1016 authorizes pharmacists to prescribe and dispense medications for HIV PrEP. Although the bill addresses minimal requirements for the pharmacist’s participation in the statewide protocol, the bill defers implementation of specific considerations to the Colorado State Board of Pharmacy, in conjunction with the state’s medical and nursing boards. The output of this multiboard collaboration is CO SBOP Rule 17 Appendix C, a protocol that specifies assessment of HIV status and high-risk behaviors, determination of PrEP eligibility (including pretreatment laboratory testing), and selection and provision of FDA-approved/CDC-recommended oral antiretroviral agents for appropriately identified PrEP candidates.
The protocol further delineates requirements for on-PrEP monitoring (laboratory and patient assessments; Table 9), minimum counseling components, dispensing guidelines, and referral processes. Patient counseling must be provided at the time of each prescription refill or interval evaluation/lab work visit: counseling components include proper medication use, including dosage, schedule; possible side effects (both common and severe); and the importance of medication adherence. Signs and symptoms of acute HIV infection, consistent use of condoms, other STI prevention techniques, and primary care follow-up for usual care should also be addressed routinely.
A positive HIV screen should prompt immediate linkage to the patient’s PCP or HIV specialist for confirmatory testing and subsequent initiation of comprehensive ART, if positive. Further, if at any time in the PrEP care continuum the patient tests positive for or develops signs/symptoms of any of the conditions listed in Table 7, appropriate referral should be made (Table 10).
Developing relationships with physicians and advanced practice nurses in the local area who are experienced in HIV care and prevention will expedite the referral process and ease patient concerns. If you, the pharmacist, are unaware of any appropriate providers in the local area, the following CDPHE resource may be of help: https://cdphe.colorado.gov/living-with-hiv.65
CO HB Bill 20-1061 and subsequent authorizations under CO State Board of Pharmacy Rule 17 Appendix C have paved the way for clinical and community pharmacists to assume active roles in HIV prevention through provision of comprehensive PrEP services (See page 28 for Appendix C in its entirety.)
PEP 101
PEP: Pharmacologic Considerations: Efficacy and Safety
HIV postexposure prophylaxis—PEP— utilizes specific combinations of antiretroviral agents to prevent infection in appropriately identified and evaluated HIV-negative people who have had a single high-risk HIV exposure.9,66 Prescribing PEP should be considered an emergency intervention: PEP is not a substitute for appropriate, continuous use of PrEP in conjunction with behavioral risk reduction. It can be thought of as similar to dispensing the morning-after contraceptive pill. PEP is most effective when initiated as soon as possible—no more than 72 hours—after a potential HIV exposure.66
Occupational HIV exposure refers to high-risk events occurring in a health care setting, whereas nonoccupational HIV exposure refers to an isolated exposure to potentially infectious body fluids that occurs outside of a health care setting, most commonly through sexual activity or injection drug use. This discussion addresses nonoccupational PEP (nPEP) only.66
Although TDF and FTC are used in PEP, the clinical/pharmacologic concerns—and intensity of treatment—are more similar to treatment of established HIV infection than to PrEP. PEP requires the use of either an integrase strand transfer inhibitor (INSTI) or a boosted protease inhibitor (PI) in combination with both TDF and FTC. Current CDC PEP guidelines do not include use of TAF as a PEP-regimen component.66
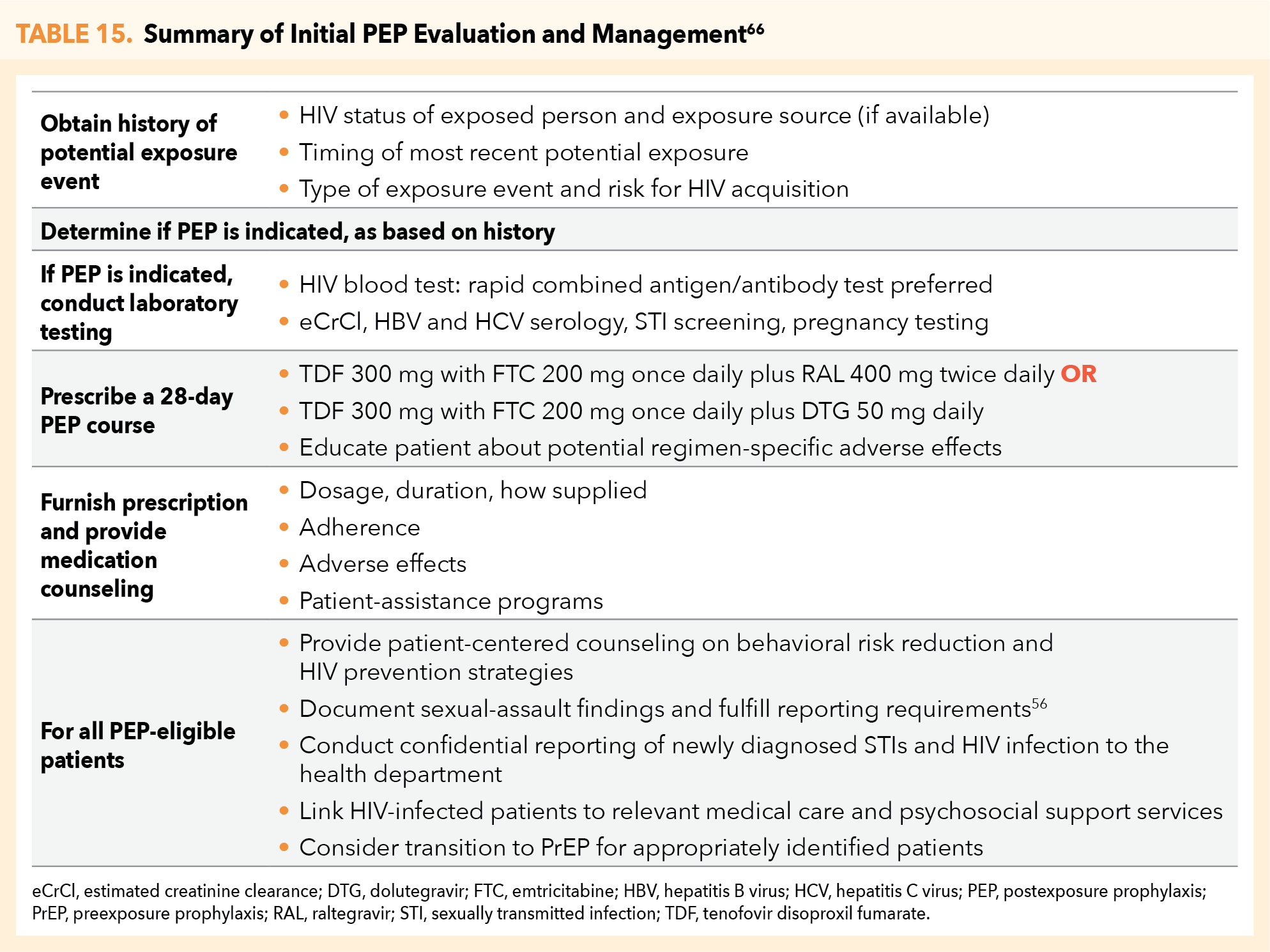
PEP is usually prescribed as a 28-day course of treatment. Currently recommended (preferred) ART regimens for use as PEP in HIV-negative adults and adolescents include the following:
- TDF 300 mg/FTC 200 mg once daily plus raltegravir (RAL) 400 mg twice daily
OR
- TDF 300 mg/FTC 200 mg once daily plus dolutegravir (DTG) 50 mg once daily
The following regimen is not approved for use under CO SBOP Rule 17, although it is included under the CDC guidelines as an alternative: TDF 300 mg/FTC 200 mg once daily plus darunavir (DRV) 800 mg and ritonavir (RTV) 100 mg once daily.2,66
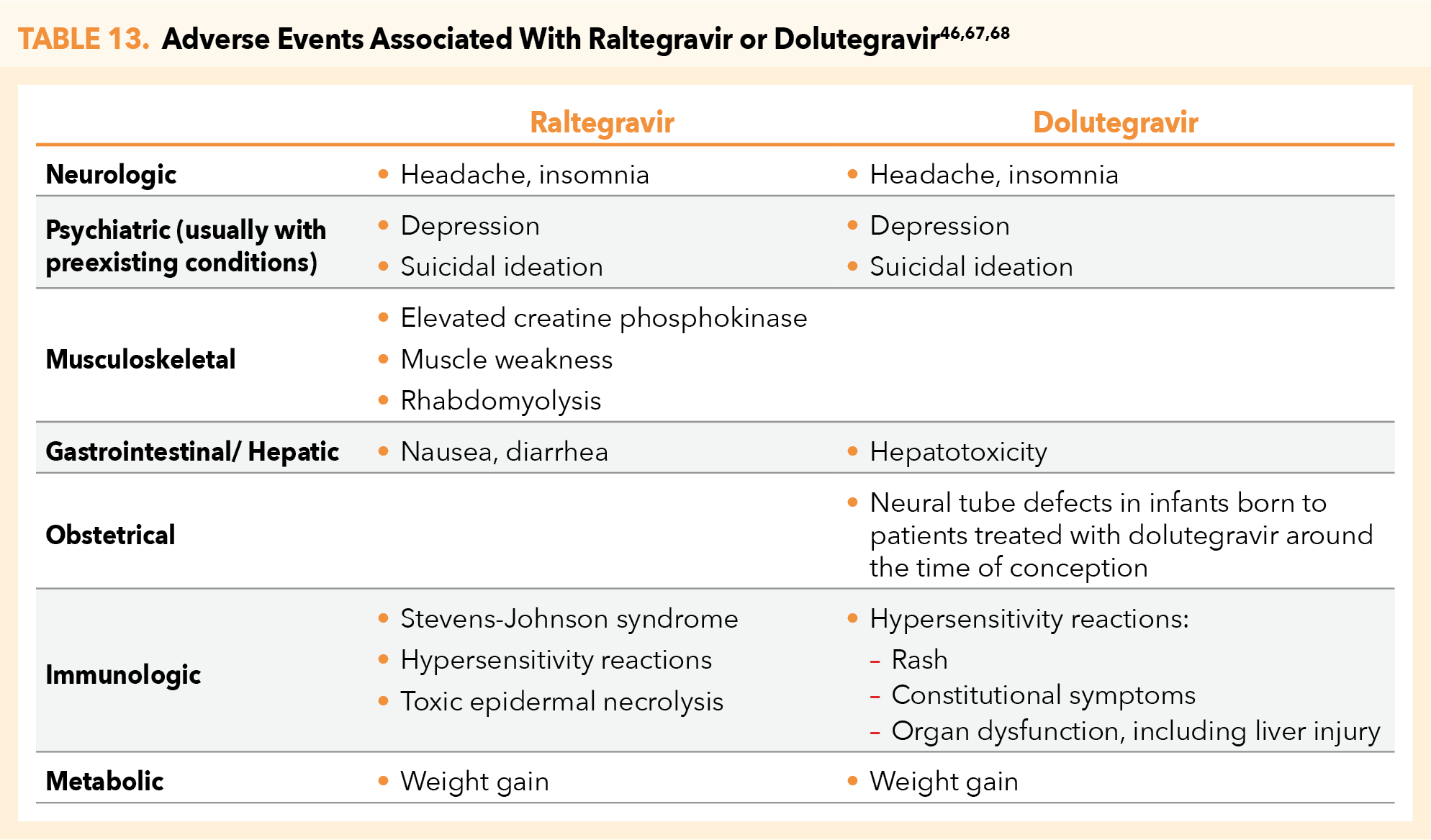
The CDC PEP guidelines do not in general recommend 1 INSTI over the other (ie, DTG vs RAL), however, DTG may be preferred for its once-daily dosing.66-68 Note that use of DTG should be avoided in periconceptionperiod because of the risk of neural tube defects linked to drug exposure during the first 28 days of gestation, yet there are no data to suggest that DTG has any teratogenic signal after the periconception period.69 Similarly, nonpregnant women of childbearing potential who are sexually active or have been sexually assaulted and are not using effective birth control should not receive DTG.68 In either clinical scenario, patients should be prescribed RAL. Additional pharmacologic considerations when prescribing RAL or DTG as PEP are outlined in Table 12.49,67,68
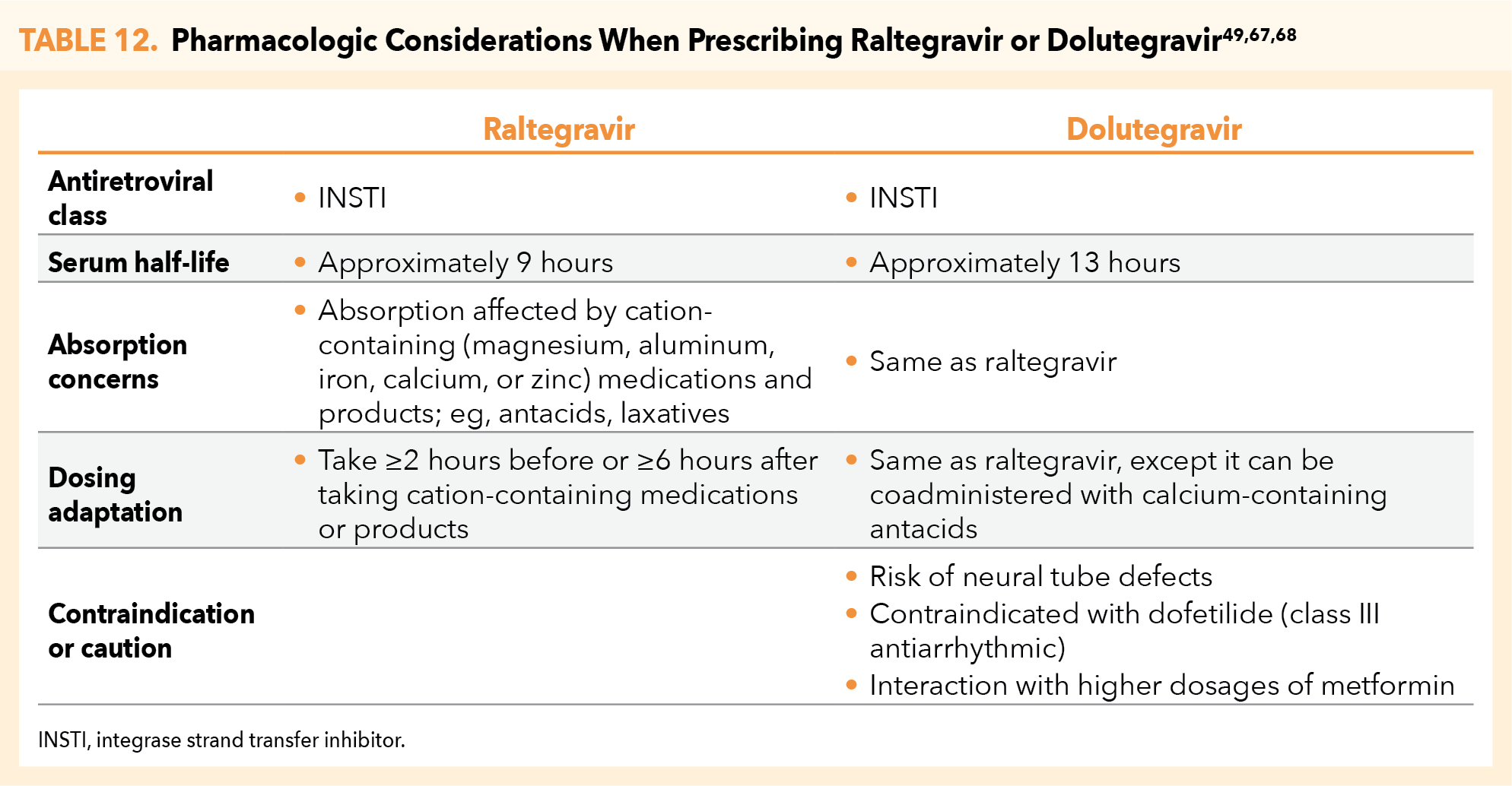
RAL and DTG are both associated with adverse events that include headache, insomnia, and weight gain.49,67,68 Both agents are also rarely associated with depression and suicidal ideation, although this usually occurs in patients who have preexisting psychiatric conditions. RAL may additionally cause fever, nausea, and diarrhea, and may be associated with more severe conditions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and rhabdomyolysis.67,68
DTG is associated with additional adverse effects that include hepatotoxicity, hypersensitivity reactions (including rash, constitutional symptoms, organ dysfunction, and liver injury) and, as noted, potential for increased risk of neural tube defects in infants born to patients treated with DTG surrounding the time of conception (Table 13).68
Pretreatment Determination of PEP Eligibility2,66
As with HIV PrEP, HIV PEP prescribing is associated with several pretreatment requirements:
- Conduct a comprehensive behavioral-risk assessment (sexual; injection drug use) and identify individuals at substantial risk of HIV acquisition
- Confirm HIV-negative status: select preferred HIV tests and/or accurately interpret HIV test results
- If a rapid test is not available and nPEP is otherwise indicated, therapy should still be initiated
- Order appropriate laboratory tests to assess for PEP-associated risks
- Screen for drug/drug interactions
- In Colorado, the patient must be 13 years of age or older
As PEP is most effective when initiated as soon as possible—no more than 72 hours—after a potential HIV exposure, these pretreatment evaluations should be performed immediately. In addition, PEP is not indicated if patient has been consistently adherent to PrEP.66
Comprehensive Sexual (and Injection Drug Use) Risk Assessment66
During the initial postexposure evaluation, the pharmacist should determine the following:
- Route/source of the potential HIV exposure
- Timing and characteristics of the exposure, including sexual assault
- Frequency of potential HIV exposures
- Risk of other STIs (gonorrhea, chlamydia, syphilis), HBV, or HCV
- Potential for current pregnancy (with pregnancy testing)
Needle-sharing during injection drug use and receptive anal intercourse are considered the highest per-act risks for nonoccupational HIV transmission. Insertive anal intercourse, insertive penile-vaginal intercourse, and oral sex carry relatively lower risks.66 Figure 7 is an algorithm for determining substantial HIV exposure. The information obtained through the comprehensive risk assessment will inform your use of the algorithm.
Confirmation of HIV-Negative Status2,66
HIV-testing considerations, as outlined (see page10) for pre-PrEP assessment—PrEP 101, Confirmation of HIV-Negative Status—also pertain to pre-PEP assessment. Special emphasis should be placed on clinical correlation of HIV-testing results and screening for signs and symptoms suggestive of acute/primary HIV infection (Table 7). Importantly, if HIV test results are unavailable during the initial evaluation, initiation of PEP should be determined on the initial assumption that the potentially exposed patient is not infected. When the HIV status of the potential source of exposure is unknown—if possible—that individual should consent to clinical evaluation and HIV testing using a 4th-gen antigen/antibody test. PEP may be considered and prescribed if an HIV test is not available or if the HIV status of the source is unknown.
Other Baseline Labs and Assessment for PEP-Associated Risks
As with HIV PrEP, pre-PEP laboratory assessment must be performed prior to treatment initiation. Again, critical labs include estimated creatinine clearance (eCrCl), HBV serology, and pregnancy testing. Additionally, if indicated by the comprehensive risk assessment, patients considered for PEP should be referred for timely STI testing and treatment. When appropriate, the patient should be offered routine prophylaxis for gonorrhea, chlamydia, or trichomoniasis. This is also an opportunity to address the need for routine vaccinations, eg, HAV, HBV, and human papillomavirus (HPV).66
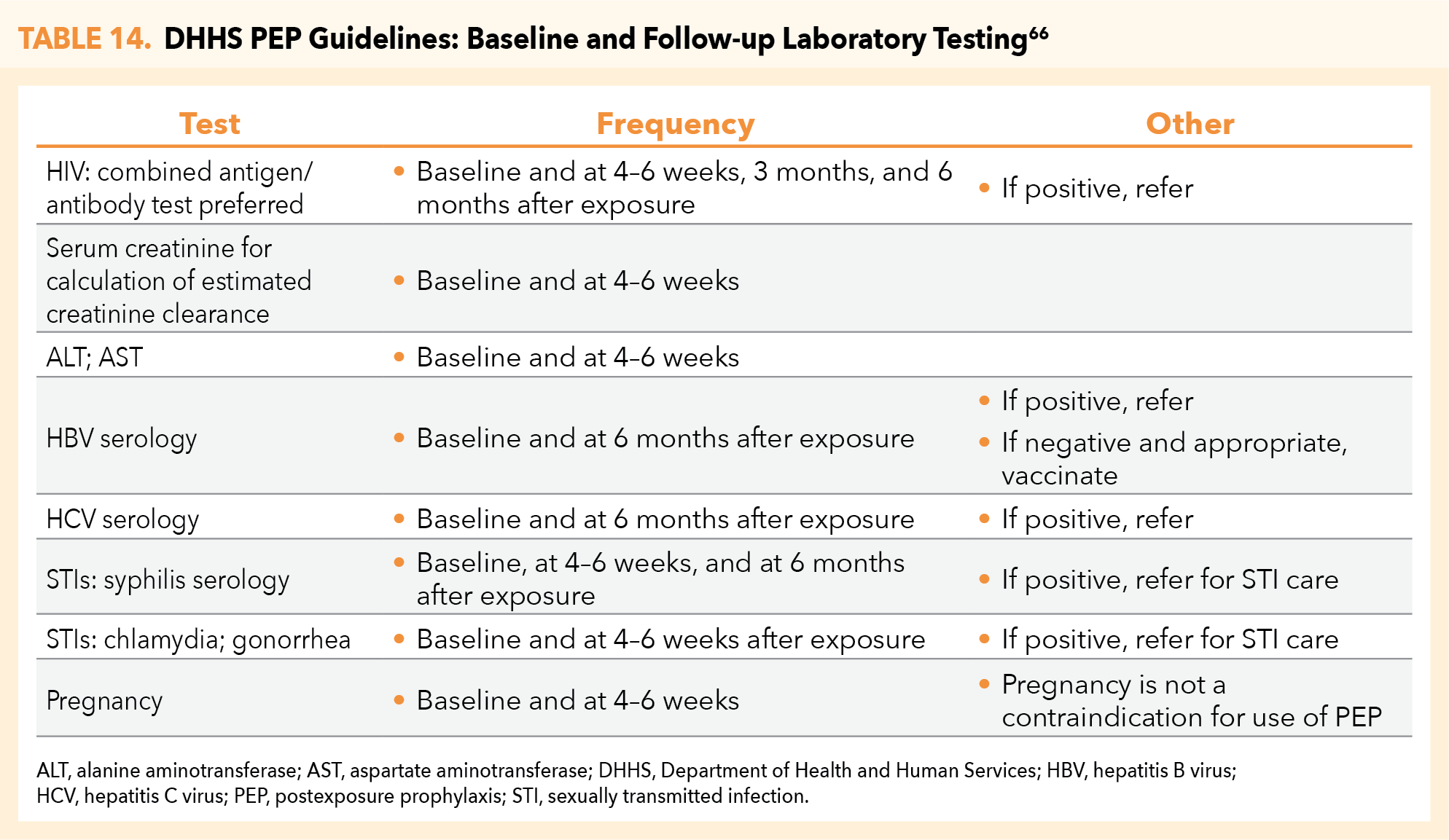
DHHS guidelines for pre-PEP (baseline) and follow-up laboratory testing largely replicate those for PrEP initiation and continuation. Differences include the addition of liver function evaluation (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and test-specific requirements for postexposure follow-up testing (Table 14).66
Patient-Centered PEP: Regimen Selection and Pretreatment Counseling6,68
Having established the patient’s PEP eligibility—or having done so to the pharmacist’s satisfaction, if HIV results are not immediately available—the PEP provider’s additional pre-PEP requirements include the following clinical activities.2
- Prescribe safe and effective PEP regimens for eligible persons without HIV infection
- Educate patients about the selected PEP regimen to optimize safe use
- Provide counseling and effective contraception to women initiating PEP who do not wish to become pregnant
- Discuss emergency contraception with women of reproductive potential
- Refer immediately for assessment of any reported sexual assault
- Provide other patient-centered counseling regarding the following:
- Medication-adherence to achieve and maintain protective tissue levels
- HIV risk reduction; including discussion and provision of prevention services referrals
- PEP medication safety during pregnancy and breastfeeding, if indicated
Basic medication-education components include the following:
- Which PEP medication is being prescribed? How is it taken? What is the dosing schedule?
- How to manage missed doses
- Common signs and symptoms suggestive of acute HIV infection (Table 7)
- Common side effects associated with raltegravir and dolutegravir (Table 13)
- What quantity will be dispensed?
A 28-day course of an INSTI-based 3-drug antiretroviral regimen should be prescribed for all persons requiring PEP. To optimize adherence, consideration should be given to patient-specific characteristics, number of doses per day and/or pills per dose, and minimization of side effects. Providing an entire 28-day course of the PEP regimen and scheduling an early follow-up visit may increase the likelihood of treatment adherence, especially if patients cannot return for multiple follow-up visits. Although 3–7-day PEP starter packs exist, they are not an option in Colorado.
Follow-up visits allow pharmacists to provide further patient-centered evaluation, education, and counseling:
- Discuss baseline blood test results (including HIV results, if a rapid test was not available at the time of initial assessment)
- Assess for adherence and adverse effects, or change the PEP regimen, if indicated
- Recommend medications for symptomatic relief of side effects (eg, antiemetics)
- Recommend pill boxes or other medication adherence aids (including smartphone apps)
- Identify strategies for dosing in line with a patient’s daily schedule
- Ensure means for patient-pharmacist contact during PEP treatment
Patients should be counseled repeatedly on the importance of PEP-regimen adherence and treatment completion to optimize prevention of HIV infection.66 Colorado Rule 17 requires pharmacists to make “every reasonable effort” to follow up with patients at 4–6 weeks post PEP initiation for determination of post-PEP HIV status. If negative, the patient should be advised that repeat HIV testing is recommended at 3 and 6 months. Further, the patient should be counseled regarding the availability of PrEP. Patients testing positive for HIV after the course of PEP has been completed should be referred to an HIV treatment specialist to transition to HIV treatment.2,66
Transitioning From PEP to PrEP
PEP is indicated only for patients with ≥1 known or suspected high-risk, unprotected HIV exposure who are HIV-negative at initial PEP evaluation. If the patient has documented HIV-negative status (preferably using a 4th-gen antigen/antibody test) upon completion of a 28-day course of PEP, s/he can be transitioned from PEP to PrEP without any gap in treatment.34,66 Patients who are at frequent, recurrent risk of HIV exposure, or require sequential or near-continuous courses of PEP, should be offered 1 of the 2 FDA-approved PrEP ART regimens in conjunction with behavioral risk-reduction counseling and interventions. Patients should receive full PrEP education and be reassessed and counseled regarding medication adherence—despite their successful completion of PEP.66
PEP Failure and Transitioning From PEP to HIV Treatment as Prevention66
If use of PEP fails to prevent HIV infection, patients may experience signs and symptoms of acute/primary, or early, HIV.33 Patients should be instructed during the initial PEP evaluation about such manifestations, as listed in Table 7. Should any signs and symptoms related to acute HIV infection occur during the 28-day PEP course or anytime within 1 month after PEP completion, patients should return for further evaluation and referral to an HIV treatment specialist, if indicated. If a patient is taking 3-agent PEP ART at the time of detected infection, ART should be continued until patient evaluation and treatment planning with an experienced HIV treatment specialist are completed.
Summary of Colorado State Board of Pharmacy Statewide Protocol (Rule 17 Appendix C) Regarding PEP2
As with PrEP, HB 20-1061 authorizes pharmacists to prescribe and dispense medications for HIV PEP. Colorado State Board of Pharmacy Statewide Protocol Rule 17 Appendix C operationalizes this authority and provides specific guidance.
Appendix C allows pharmacists to prescribe a complete 28-day course of an FDA-approved/CDC-recommended PEP antiretroviral regimen for patients meeting CDC-guideline criteria. Pharmacists must address additional patient-centered considerations beyond those assessed in the context of pre-PrEP assessment, given that high-risk HIV exposures may involve sexual abuse and/or assault.
Appendix C delineates patient assessment, laboratory testing, and counseling requirements for all phases of PEP-related care, including but not limited to pretreatment determination of PEP eligibility, PEP regimen selection, treatment adherence support, posttreatment reassessment of HIV-status, and patient-centered education regarding topics such as manifestations of acute HIV infection, availability of emergency contraception, behavioral risk mitigation, and post-PEP use of PrEP for patients remaining HIV-negative but at substantial risk of HIV acquisition. Table 15 provides a consolidated summary of initial PEP evaluation and longitudinal management.
Hand-in-hand with Colorado-based pharmacists’ prescribing and management of HIV PrEP, the PEP protocol allows for the delivery of comprehensive HIV prevention services (See page 28 for Appendix C in its entirety.)
Embracing Sexual Wellness
The Sexual Health Assessment
A comprehensive sexual health assessment is critical to determining an individual’s need for PrEP, as HIV risk may be associated with numerous personal, partner, relationship, social, cultural, network, and community factors.34 Many health care providers do not ask about same-sex behaviors because of personal discomfort or anticipated patient discomfort. Further, patients often do not disclose sexual behaviors because of perception of stigma or fear of judgment. Frank, nonjudgmental questions about sexual behavior, alcohol use, and illicit drug use should be asked.34 The CDC’s sexually transmitted diseases treatment guidelines recommend the following risk-behavior questions, known as The 5 Ps70:
- Partners: In the past 12 (or 3, or 6) months, with how many partners have you had sex? Are they men, women, transgender women, and/or transgender men?
- Practices: To understand your risks for STIs, I need to understand the kind of sex you have had recently. Do you have oral sex, vaginal sex, anal sex? For MSM: are you a top (insertive), bottom (receptive), or versatile (insertive or receptive)?
- History of STIs: Have you ever had an STI? When? Have any of your partners had an STI?
- Protection from STIs: How often do you use condoms? What do you do to protect yourself from HIV and STIs?
- Pregnancy plans: Do you wish to prevent pregnancy? Are you using contraception; if so, what type?
Clinicians can enhance their history-taking skills by making patients feel more comfortable and, therefore, potentially more open: use neutral and inclusive terms; avoid assumptions based on age, appearance, marital status, or other factors; and ensure that patients share an understanding of the terms used. (Figure 8).71-73 For additional training on opening a sexual health dialogue, go to https://realcme.com/learner/course/2591 or https://realcme.com/learner/course/2508.
Before starting, consider informing the patient about the routine nature of conducting a sexual-health assessment with the following statement: “I am going to ask you a few questions about your sexual history. I ask these questions at least once a year of all my patients because the information is very important for your overall health. Everything you tell me is confidential, as part of our patient-provider relationship. Do you have any questions before we start?” If patients want to know the reasons/importance of a sexual health assessment, consider using the following statements74:
- Sexual health is important for overall emotional and physical health
- We ask these questions every year because it is common for people’s sexual behaviors and partners to change over time
- As you may know, sexual activity without protection can lead to STIs. These diseases—especially syphilis, gonorrhea, and chlamydia—are very common and often do not cause any symptoms. If we do not catch and treat these diseases, they can affect general health and well-being
- These questions can also help guide our conversation about how you can protect yourself from STIs, unwanted pregnancy, or other issues that may concern you. It will also give you an opportunity to talk about problems with, or changes in, sexual desire and functioning
- Our discussion may help us identify other health needs (eg, additional laboratory testing; vaccinations) or resources you may need as part of your lifestyle (sexual- or nonsexual-related)
Communicating With Transgender Persons
In addition to the empathy and respect that you show all patients participating in sexual-history discussions, there may be special considerations when taking sexual histories with transgender people, a high-risk population that is underrepresented among PrEP users. The following points are taken from the National LGBTQIA+ Health Education Center and may also be helpful when taking sexual histories from other individuals, especially with regard to gender pronouns.71
- Make sure you have established a good rapport with the patient before asking about sexual practices (or doing a physical exam)
- Be sure to use the patient’s preferred name during conversations. This will not necessarily be the name that appears on insurance and medical records
- Ask what pronoun(s) your patient prefers. This is best done on an intake form but may also be needed during the clinic visit. Some people change their personal pronoun preference
- Many transgender people want you to use the pronoun that matches their gender identity. For example, a transgender woman would like you to use “she/her”
- Some transgender or gender-nonconforming people may ask you to use “ze” or “they,” or to try to avoid using any pronouns
Discussing Risk of HIV Acquisition Through Injection Drug Practices
The USPSTF recommends that all health care providers be aware of signs and symptoms associated with injection drug use. Brief risk-behavior assessment questions that address this concern include the following34:
- Have you ever injected drugs that were not prescribed to you by a clinician?
- (If yes), When did you last inject nonprescribed drugs?
- In the past 6 months, have you injected using needles, syringes, or other drug-preparation equipment that had already been used by another person?
- In the past 6 months, have you been in a methadone or other medication-assisted substance use disorder treatment program?
Answers from these questions may help you determine if additional resources are needed for patients who inject drugs or have other high-risk substance-use related concerns or behaviors. Such resources include harm-reduction (behavioral risk-reduction) programs and services, medication-assisted treatment (eg, buprenorphine), inpatient or residential drug treatment programs, or relapse-prevention services (eg, 12-step programs or mental health/behavioral support programs).34,62 The website for the Substance Abuse and Mental Health Services Administration (https://www.samhsa.gov) is a valuable resource for locating substance use disorder treatment facilities and programs and behavioral and mental health services.75
Helping Your Patient Navigate Beyond Pharmacy-Based HIV Preventive Care: Referral, Advocacy & Resources
When to Refer to Primary Care or Specialist Providers
A positive HIV screen should prompt immediate linkage to the patient’s PCP or HIV specialist for confirmatory testing and subsequent initiation of comprehensive ART, if positive.
Further, if acute HIV is suspected (based on symptoms)—or a patient tests positive for HBV, becomes pregnant, or has symptoms or laboratory findings of acute renal injury—a referral to a PCP or other appropriate provider should be made and the patient should cease participation in the statewide protocol. A positive STI screen or considerations pertaining to usual care are not necessarily reasons to terminate a patient’s participation.
Developing relationships with physicians and advanced practice nurses in your area who are experienced in HIV care and prevention will expedite the referral process and ease patient concerns. The individual patient’s records, along with full documentation of pharmacist-provided PrEP services, should be transferred to the patient’s PCP, whenever a referral is necessary.
Patients who do not have a PCP experienced in PrEP provision can be referred to the American Academy of HIV Medicine database to locate a PrEP provider in their area76: https://providers.aahivm.org/referral-link-search?reload=timezone. Pharmacists can also locate appropriate providers using the following CDPHE resource: https://cdphe.colorado.gov/living-with-hiv.65
Keep in mind that patients who initially request PrEP or PEP from their pharmacist—not their PCP—may have a higher level of comfort with and/or do not perceive stigma/discrimination on the part of the pharmacist. This trusted patient-pharmacist relationship should be maintained to support the patient’s well-being.
Your Patient’s Advocate: Financial-Assistance Resources
CO HB 20-1016 not only expands the pharmacist’s scope of practice to include prescribing and dispensing PrEP and PEP, but also addresses equitable reimbursement by insurance carriers for all pharmacist-directed PrEP/PEP services.1
For those patients who lack prescription drug coverage, several resources are available. Ready, Set, PrEP is a nationwide program launched by the CDC in December 2019 to make PrEP available at no cost to individuals who meet the following requirements2,10:
- Test negative for HIV AND
- Have a valid prescription from a health care provider AND
- Do not have health insurance coverage for outpatient prescription drugs
Although Ready, Set, PrEP ensures access to PrEP for qualifying individuals, it also stipulates that the costs for necessary clinic visits and laboratory testing may vary based on income.10 Gilead’s Advancing Access® program is another patient assistance program available for both insured and uninsured patients: http://www.gileadadvancingaccess.com.76
Uninsured/underinsured patients and providers can visit the following CDPHE webpage for additional information: https://cdphe.colorado.gov/prep-for-hiv.78 Further, CDPHE offers guidance on reimbursement of pharmacists who do not have insurance-carrier contracts covering PrEP/PEP services: https://cdphe.colorado.gov/public-health-intervention-program.79
Pharmacist Resources
The CDC has created tools to help pharmacists and other providers keep track of important patient and provider steps before PrEP is prescribed62: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2017.pdf.
Establishing a safe and supportive environment in which to discuss a patient’s personal health issues—one that also promotes confidentiality—is an important consideration for pharmacists who are starting to build a PrEP/PEP-provider practice.80 Several resources are available to help pharmacists develop a comprehensive and supportive sexual-history dialogue with patients:
Addressing Practical Challenges of PrEP/PEP Implementation
The Colorado model allows any qualified pharmacist in the state to offer PrEP/PEP services independently of physician authorization and is intended to help fill gaps in care, especially in urgent situations where a PCP is not established. Colorado’s HB 20-1061 protocol requires all pharmacies/pharmacists to participate in accordance with the statewide regulations, which ensure a standard level of care across all pharmacies providing the service.1,2
Other models in the United States utilize a collaborative practice agreement that reflects an established relationship among providers (eg, pharmacists, physicians, and advanced practice nurses) and generally requires a referral. Mission Wellness Pharmacy is a one-stop PrEP program that allows pharmacists to initiate and furnish PrEP directly to patients as part of a demonstration project with the San Francisco Department of Public Health.81 Kelley-Ross Pharmacy in Seattle is able to offer pharmacist-driven PrEP provision and HIV-prevention services through not only collaborative practice agreements, but also through Washington State legislation allowing pharmacists to bill for patient-care services, including monthly patient evaluations, without a limit on length of time the patient can remain under the care of a pharmacist.82
Both models of care are beneficial to expanding preventive services and HIV prevention.
Building In-Pharmacy Infrastructure and Capacity
A commentary in the Journal of the American Pharmacists Association (2020) provides recommendations for implementing a community-based PrEP program, one reflecting the previously discussed San Francisco and Seattle models. That publication also provides recommendations for how community pharmacies can integrate PrEP/PEP implementation with their existing services infrastructure83:
- Set up logistics for on-site laboratory testing or for sending out specimens
- Provide designated physical space (including restrooms) within the pharmacy for laboratory specimen collection
- Order CLIA-waived rapid HIV tests
- Utilize mail-in lab kits
- Train staff regarding on-site (“one-stop PrEP”) and/or off-site specimen collection and handling
- Consider use of an on-site phlebotomist.
Note: Colorado does not have a licensing body for phlebotomy or specified requirements for conducting sample collection; therefore, it is possible to train existing staff to perform this duty
- Establish a relationship with an external lab; using a single lab can help streamline staff training and administrative burden
- Work with your lab of choice to discuss the feasibility of its providing designated staff at your collection site. Many labs with significant capacity can offer this service
- Train staff on self-collection (patient) techniques and post instructions with visual aids in the restroom. If onsite specimen collection is not an option for your practice location, work with a single lab with several specimen collection locations
- Manage laboratory test results
- Adapt pharmacy workflow
- Encourage an appointment-based model for protocol-based assessment, laboratory specimen collection, and prescription pick-up
- Establish private/confidential spaces for discussion of test results
- Consider installing modular-ready private counseling rooms
- Assign specific pharmacy staff to accommodate walk-in requests for lab results
- Establish communication protocols and methods
- Determine how confidential information will be shared among team members, patients, referring providers, or health departments in accordance with Health Insurance Portability and Accountability Act (HIPAA) requirements84
- Set up online portals with secure access for e-mail, text messages, apps, and shared electronic medical records (EMRs); this can save both time and staff resources
- Utilize secure lab-based online portals for accessing test results; access eligibility may vary
- Create a standardized referral mechanism for positive screens or abnormal laboratory results
- Provide and monitor pharmacist education and hands-on training, including training of pertinent auxiliary pharmacy staff
- Require pharmacists to demonstrate competency in delivering PrEP and PEP per state and national guidelines, inclusive of sexual-health counseling, comprehensive risk-appropriate prevention strategies, laboratory testing and interpretation, and navigation of insurance benefits and patient-assistance programs
- Set realistic time frames for implementation of new work processes and attainment of target number of patient visits
- Provide ongoing pharmacist and staff feedback, training, and monitoring
- Designate a pharmacy staff member to disseminate PrEP/PEP guideline changes
Part of ensuring successful PrEP/PEP services in the community-pharmacy setting is engaging and supporting pharmacists who can educate stakeholders, build community-based relationships and partnerships, and break down barriers to PrEP access.81,85 Ultimately, instead of waiting to access PrEP, patients will be able to participate in a 45-minute conversation with the pharmacist to get the PrEP they need—when they need it.
Concluding Comments
HIV Prevention: The Pharmacist as Educator, Provider, and Advocate
This monograph has outlined the Colorado pharmacist’s role in the provision of HIV PrEP and PEP, as based on CO HB 20-1061 and subsequent authorizations under CO State Board of Pharmacy Rule 17 Appendix C. A comprehensive statewide protocol allowing pharmacists to provide nationally endorsed public health preventative care is a win for patient access to care and a strike against the spread of HIV. However, success of this model of care will depend on overcoming implementation challenges. HB 20-1061 has made a significant first step to ensure sustainability of advanced-level clinical services through both direct billing and equitable reimbursement, but the full implementation of a pharmacy infrastructure that supports the provision of PrEP/PEP will remain a challenge for many.1,2
The pharmacy setting is an ideal entry point for people at risk of HIV acquisition to initiate pre- or postexposure HIV prophylaxis. The pharmacist’s role as HIV-prevention educator, PrEP/PEP provider, and patient advocate has the potential to grow into a greater presence within the local public health sphere. This expansion of the pharmacist’s scope of practice can potentially help build strong relationships with patients, PCPs, and community-based patient-support services, while establishing the pharmacist as an essential and reliable resource. Provision of PrEP and PEP services by well-trained and well-placed pharmacists can be a driving force in attaining the goal of zero new HIV infections.
List of Abbreviations
Ab antibody
Ag antigen
AIDS acquired immunodeficiency syndrome
ALT alanine aminotransferase
AST aspartate aminotransferase
ART antiretroviral therapy
CAB cabotegravir
CDC Centers for Disease Control and Prevention
CDPHE Colorado Department of Public Health & Environment
CI confidence interval
CLIA Clinical Laboratory Improved Amendments
DAA direct-acting antiviral
DDI drug-drug interaction
DHHS US Department of Health and Human Services
DNA deoxyribonucleic acid
DRV darunavir
DTG dolutegravir
EBR/GZR elbasvir/grazoprevir
eCrCl estimated creatinine clearance
EMR electronic medical record
GLE/PIB glecaprevir/pibrentasvir
FDA US Food and Drug Administration
FEM-PrEP Preexposure Prophylaxis Trial for HIV Prevention Among African Women
FTC emtricitabine
Gen generation
HAV hepatitis A virus
HBV hepatitis B virus
HCV hepatitis C virus
HDL high-density lipoprotein cholesterol
HIPAA Health Insurance Portability and Accountability Act
HIV human immunodeficiency virus
HPV human papillomavirus
IA immunoassay
IgG immunoglobulin G
IgM immunoglobulin M
IAS–USA International Antiviral Society–USA
IDU injection drug use
iPrEx Iniciativa Profilaxis Pre-Exposición [Preexposure Prophylaxis Initiative]
INSTI integrase strand transfer inhibitor
IRR incidence rate ratio
LDL low-density lipoprotein
MSM men who have sex with men
NAAT nucleic acid amplification testing
NNRTI nonnucleoside reverse transcriptase inhibitors
NRTI nucleoside reverse transcriptase inhibitors
NSAID nonsteroidal anti-inflammatory drug
NI noninferiority
NIH National Institutes of Health
nPEP nonoccupational PEP
PCP primary care provider
PCR polymerase chain reaction
PEP postexposure prophylaxis
PI protease inhibitor
PWH/PLWH people with HIV/people living with HIV
PrEP preexposure prophylaxis
PY person-years
PWID people who inject drugs
RAL raltegravir
RNA ribonucleic acid
RTV ritonavir
SBOP State Board of Pharmacy
SOF/LDV sofosbuvir/ledipasvir
SOF/VEL sofosbuvir/velpatasvir
STI sexually transmitted infection
TAF tenofovir alafenamide
TDF tenofovir disoproxil fumarate
TDF2 Botswana TDF/FTC Oral HIV Prophylaxis trial
WHO World Health Organization
REFERENCES
- State of Colorado. House Bill. 20-1061. July 13, 2020. https://leg.colorado.gov/bills/hb20-1061. Accessed January 21, 2021.
- Colorado Department of Regulatory Agencies. State Board of Pharmacy: Laws, Rules and Policies. Code of Colorado Regulations 3 CCR 719-1. Rule 17 Appendix C. 2021. https://dpo.colorado.gov/Pharmacy/Laws. Accessed January 21, 2021.
- Centers for Disease Control and Prevention (CDC). HIV Basics: US Statistics. Updated June 30, 2020. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics. Accessed January 21, 2021.
- Policy, Planning and Strategic Communication. Dear colleagues: information from CDC’s Division of HIV/AIDS Prevention. https://www.cdc.gov/hiv/policies/dear-colleague/dcl/050720.html. Accessed January 21, 2021.
- Roser M, Richie H. HIV/AIDS. Last updated November 2019. https://ourworldindata.org/hiv-aids. Accessed January 21, 2021.
- Kochanek KD, et al. Deaths: final data for 2017. Natl Vital Stat Rep. 2019;68(9):1-77.
- US Department of Health & Human Services (DHHS). What Is Ending the HIV Epidemic: A Plan for America? October 2020. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed January 21, 2021.
- Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS (Auckl). 2012;4:117-124.
- Pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP). Updated September 2020. https://www.cdc.gov/hiv/clinicians/prevention/prep-and-pep.html. Accessed January 21, 2021.
- Ending the HIV epidemic: Ready, Set, PrEP. Updated November 2020. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/prep-program. Accessed January 21, 2021.
- Choopanya K, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090.
- Anderson PL, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125.
- Kazi DS, et al. PrEParing to end the HIV epidemic—California’s route as a road map for the United States. N Engl J Med. 2019;381(26):2489-2491.
- Hess KL, et al. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238-243.
- Goldstein RH, et al. Being PrEPared—preexposure prophylaxis and HIV disparities. N Engl J Med. 2018;379(14):1293-1295.
- Siegler AJ, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841-849.
- HIV. Figures. Diagnoses of HIV infection in the United States and Dependent Areas, 2018—United States and 6 dependent areas. https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-31/content/figures.html. Accessed January 21, 2021.
- Cahill S, et al. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2017;29(11):1351-1358.
- Highleyman L. First generic Truvada now available in the United States. POZ Online. October 2, 2020. https://www.poz.com/article/first-generic-truvada-now-available-united-states. Accessed January 21, 2021.
- Life-saving naloxone from pharmacies. Updated August 6, 2019. https://www.cdc.gov/vitalsigns/naloxone/index.html. Accessed January 21, 2021.
- Newman TV, et al. Optimizing the role of community pharmacists in managing the health of populations: barriers, facilitators, and policy recommendations. J Manag Care Spec Pharm. 2019;25(9):995-1000.
- Kibicho J, Owczarzak J. Pharmacists’ strategies for promoting medication adherence among patients with HIV. J Am Pharm Assoc (2003). 2011;51(6):746-755.
- Hill LA, et al. The role of the clinical pharmacist in the management of people living with HIV in the modern antiretroviral era. AIDS Rev. 2019;21(4):195-210.
- Tseng A, et al. Role of the pharmacist in caring for patients with HIV/AIDS: clinical practice guidelines. Can J Hosp Pharm. 2012;65(2):125-145.
- Brown J. As HIV infection rates rise in Colorado, pharmacies can now prescribe preventative pills. The Colorado Sun. November 18, 2020. https://coloradosun.com/2020/11/18/as-hiv-infection-rates-rise-in-colorado-pharmacies-can-now-prescribe-preventative-pills/. Accessed January 21, 2021.
- Colorado Department of Public Health and Environment (CDPHE). HIV surveillance quarterly reports. https://cdphe.colorado.gov/sexually-transmitted-infections-and-hiv/sti-hiv-data/hiv-surveillance-quarterly-reports. Accessed January 21, 2021.
- U.S. statistics: fast facts. Updated June 2020. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics. Accessed January 21, 2021.
- State of Colorado. Colorado revised statutes 2019. Title 12; 12 280 103. https://leg.colorado.gov/sites/default/files/images/olls/crs2019-title-12.pdf. Accessed January 22, 2021.
- The science of HIV and AIDS–overview. Last updated October 2019. https://www.avert.org/professionals/hiv-science/overview. Accessed January 21, 2021.
- Cummins NW, Badley AD. Making sense of how HIV kills infected CD4 T cells: implications for HIV cure. Mol Cell Ther. 2014;2:20.
- Understanding HIV/AIDS: The HIV life cycle. Updated July 1, 2019. https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/19/73/the-hiv-life-cycle. Accessed January 21, 2021.
- Liu Y, Tran N. Best practices for HIV-1/2 screening: when to test and what to test. https://blog.ucdmc.ucdavis.edu/labbestpractice/?s=best+practices+for+HIV-1%2F2+screening. Accessed January 21, 2021.
- Patel P, et al. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012;54(1):42-47.
- US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2017 Update (A Clinical Practice Guideline). March 2018. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed January 21, 2021.
- Pre-exposure prophylaxis (PrEP). Last reviewed May 2020. https://www.cdc.gov/hiv/clinicians/prevention/prep.html. Accessed January 21, 2021.
- US Food and Drug Administration (FDA). Drugs@FDA: FDA-Approved Drugs. Emtricitabine and tenofovir disoproxil fumarate (Truvada®; package insert). Last updated June 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021752s061lbl.pdf. Accessed January 21, 2021.
- Drugs@FDA: FDA-Approved Drugs. Emtricitabine and tenofovir alafenamide (Descovy®; package insert). Last updated December 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208215s011lbl.pdf. Accessed January 21, 2021.
- Grant RM, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587-2599.
- Baeten JM, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410.
- Murnane PM, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. 2013;27(13):2155-2160.
- Van Damme L, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411-422.
- Thigpen MC, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423-434.
- Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am. 2014;28(3):371-402.
- Hare CB, et al. The phase 3 DISCOVER study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Presented at Conference on Retroviruses and Opportunistic Infections (CROI) 2019. March 4–7, 2019; Seattle, Washington. Abstract: O-10.
- Ruane P, et al. Phase 3 randomized, controlled DISCOVER study of daily F/TAF or F/TDF for HIV pre-exposure prophylaxis: week 96 results. Presented at 17th European AIDS Conference (EACS). November 6-9, 2019; Basel, Switzerland. Poster: PE3/16.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
- Chan L, et al. Potential kidney toxicity from the antiviral drug tenofovir: new indications, new formulations, and a new prodrug. Curr Opin Nephrol Hypertens. 2018;27(2):102-112.
- Tao X, et al. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infect Dis. 2020;93:108-117.
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines. Accessed January 21, 2021.
- University of Liverpool. HEP Drug Interactions. https://www.hep-druginteractions.org/checker. Accessed January 21, 2021.
- Glidden DV, et al. Symptoms, side effects and adherence in the iPrEx open-label extension. Clin Infect Dis. 2016;62(9):1172-1177.
- HIV Testing. Last reviewed June 2020. https://www.cdc.gov/hiv/testing/index.html. Accessed January 21, 2021.
- Rapid HIV tests suitable for use in non-clinical settings (CLIA-waived). Updated June 11, 2018. https://www.cdc.gov/hiv/pdf/testing/rapid-hiv-tests-non-clinical.pdf. Accessed January 21, 2021.
- Landovitz RJ, et al. HPTN083 interim results: Pre-exposure prophylaxis (PrEP) containing long-acting injectable cabotegravir (CAB-LA) is safe and highly effective for cisgender men and transgender women who have sex with men (MSM,TGW). Presented at: 23rd International AIDS Conference (AIDS2020: Virtual). July 6−10, 2020; virtual. Abstract OAXLB0101.
- World Health Organization. Trial results reveal that long-acting injectable cabotegravir as PrEP is highly effective in preventing HIV acquisition in women. November 9, 2020. https://www.who.int/news/item/09-11-2020-trial-results-reveal-that-long-acting-injectable-cabotegravir-as-prep-is-highly-effective-in-preventing-hiv-acquisition-in-women. Accessed January 21, 2021.
- Saag MS, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379-396.
- World Health Organization (WHO). What is the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV for men who have sex with men: update to WHO’s recommendation on oral PrEP. July 2019. https://www.who.int/hiv/pub/prep/211/en. Accessed January 21, 2021.
- Molina J-M, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237-2246.
- PrEP. Last reviewed November 2020. https://www.cdc.gov/hiv/basics/prep.html. Accessed January 21, 2021.
- Harm Reduction Coalition. https://harmreduction.org. Accessed January 21, 2021.
- Lambers F, et al. Changing the odds: motives for and barriers to reducing HCV-related sexual risk behavior among HIV-infected MSM previously infected with HCV. BMC Infect Dis. 2018;18(1):678.
- US Public Health Service. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2017 Update. Clinical Providers’ Supplement. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2017.pdf. Accessed January 21, 2021.
- Cairns G. Does PrEP cause more HIV drug resistance? Yes, some. NAM AIDSmap. February 2020. https://www.aidsmap.com/news/feb-2020/does-prep-cause-more-hiv-drug-resistance-yes-some. Accessed January 21, 2021.
- HIV Treatment as Prevention. Last reviewed March 2020. https://www.cdc.gov/hiv/risk/art/index.html. Accessed
January 21, 2021.
- People living with HIV. https://cdphe.colorado.gov/living-with-hiv. Accessed January 21, 2021.
- Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV—United States, 2016. https://www.cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. 2016. Accessed January 21, 2021.
- Drugs@FDA: FDA-Approved Drugs. Raltegravir package insert. Last updated August 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022145s029lbl.pdf. Accessed January 21, 2021.
- Drugs@FDA: FDA-Approved Drugs. Dolutegravir package insert. June 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204790s025lbl.pdf. Accessed January 21, 2021.
- Zash R. Update on neural tube defects with antiretroviral exposure in the Tsepamo study, Botswana. Presented at: AIDS2020: Virtual. July 6-10, 2020; virtual. Abstract OAXLB0102. As reported by NAM AIDSMap. https://www.aidsmap.com/news/jul-2020/if-we-dont-include-women-trials-we-are-not-protecting-women-dolutegravir-pregnant. Accessed January 21, 2021.
- Clinical Prevention Guidance. https://www.cdc.gov/std/tg2015/clinical.htm. Accessed January 21, 2021.
- National Association of Community Health Centers, National LGBT Health Education Center. Taking Routine Histories of Sexual Health: A System-Wide Approach for Health Centers. February 2016. https://www.lgbthealtheducation.org/publication/taking-routine-histories-of-sexual-health-a-system-wide-approach-for-health-centers. Accessed January 21, 2021.
- Sexual History-Taking Toolkit. July 2020. https://targethiv.org/library/sexual-history-taking-toolkit. Accessed January 21, 2021.
- Discussing sexual health with your patients. Last reviewed October 2019. https://www.cdc.gov/hiv/clinicians/screening/discussing-sexual-health.html. Accessed January 21, 2021.
- Pakpreo P. Why do we take a sexual history? Virtual Mentor. 2005;7(10):677-682.
- Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov. Accessed January 21, 2021.
- American Academy of HIV Medicine (AAHIVM). Referral Link. https://providers.aahivm.org/referral-link-search?reload=timezone. Accessed January 21, 2021.
- Gilead Advancing Access. 2020. http://www.gileadadvancingaccess.com. Accessed January 22, 2021.
- Pre-exposure prophylaxis (PrEP). https://cdphe.colorado.gov/prep-for-hiv. Accessed January 21, 2021.
- Public health intervention program (PHIP). https://cdphe.colorado.gov/public-health-intervention-program. Accessed January 21, 2021.
- A Guide to Taking a Sexual History. https://www.cdc.gov/std/treatment/sexualhistory.pdf. Accessed January 21, 2021.
- Miller J. Increasing access to HIV prevention medicine: Healthforce Center at UCSF. November 19, 2019. https://healthforce.ucsf.edu/blog-article/healthcare-policy/increasing-access-hiv-prevention-medication. Accessed January 21, 2021.
- Tung EL, et al. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561.
- Lopez MI, et al. Community pharmacy delivered PrEP to STOP HIV transmission: an opportunity NOT to miss! J Am Pharm Assoc (2003). 2020;60(4):e18-e24.
- HIPAA for Professionals. Last reviewed June 2017.. Accessed January 21, 2021.
- Ross M. 4 findings from a successful HIV PrEP program. https://www.pharmacytimes.com/news/4-findings-from-a-successful-hiv-prep-program. Accessed January 21, 2021.