
Expired activity
Please go to the PowerPak
homepage and select a course.
Introduction
Building on a tradition of immune-focused research first introduced by William Coley in the 1890s, an evolution in cancer therapy has harnessed the power of the immune system and immune response to proactively attack and kill cancer cells.1,2 Immunotherapy, a term that encompasses a diverse class of treatment modalities (Table 1), focuses on unlocking the capacity of the existing immune response to exert a therapeutic effect against cancer.3 While the promise of immunotherapies has resulted in unprecedented response in patients with many types of cancers, these therapies, and specifically immune checkpoint inhibitors (ICIs), are also associated with a unique toxicity profile, often referred to as immune-related adverse events (irAEs).4-6 While the more common symptoms of these toxicities, such as diarrhea or skin rash, may seem familiar to cancer care clinicians, the underlying pathophysiology and management is distinct from the same symptom presentation that occurs with chemotherapy, targeted therapies, and/or radiotherapies.6 Further, the more rare irAEs may prove additionally challenging to recognize, identify, and manage. Here we present an overview of irAEs, with an emphasis on the unique pathophysiology of irAEs generally, as well as available resources to support an evidence-based approach to monitoring, identification, and management of the irAEs associated with ICI, including rare toxicities and unique patient presentations. This review focuses on some practical strategies that can help practitioners optimize patient care through counseling, education, detection and timely intervention.
| Table 1. Immunotherapeutic Approaches |
| Description |
Examples of FDA-Approved Products |
|
| Immune checkpoint inhibitors |
Drugs that work by blocking checkpoint proteins from binding to their partner proteins resulting in the inhibition of T cell deactivation. The ultimate activation of T cells |
Ipilimumab (Yervoy)
Cemiplimab (Libtayo)
Nivolumab (Opdivo)
Pembrolizumab (Keytruda)
Atezolizumab (Tecentriq)
Avelumab (Bavencio)
Durvalumab (Imfinzi) |
| T-cell transfer therapy (also called adoptive cell therapy) |
Immunotherapy that focuses on the modification of a patient’s own immune cells to better attack cancer cells. Two main types of T-cell transfer therapy include chimeric antigen receptor T cells (CAR T cells) and tumor-infiltrating lymphocytes (TILS) |
CAR T cells:
Axicabtagene ciloleucel (Yescarta)
Brexucabtagene autoleucel (Tecartus)
Tisagenlecleucel (Kymriah)
Tumor-infiltrating lymphocytes: not currently marketed in US |
| Monoclonal antibodies |
Monoclonal Antibodies:
Man-made proteins that act like human antibodies developed to target specific antigens associated with a cancer or cancer process. The mechanism of action may include increase in ADCC, CDCC or apoptosis. |
Rituximab (Rituxan)
Trastuzumab (Herceptin)
Bevacizumab (Avastin)
Note: all the ICI are monoclonal antibodies |
Monoclonal antibody drug conjugate:
Monoclonal antibodies can be combined with a chemotherapy drug to better deliver the drug to a target (eg,, a target associated with a cancer cell). |
Brentuximab vedotin (Adcetris)
Enfortumab vedotin (Padcev)
Sacituzumab govitecan (Trodelvy) |
| Vaccines |
Vaccines may be used to help prevent cancer such as vaccine against some strains of human papillomavirus that are linked to a number of cancers including cervical and head and neck cancer.
Therapeutic cancer vaccines are developed to increase the immune response to cells with one or more specific antigen. |
Sipuleucel-T (Provenge)
Talimogene laherparepvec (T-VEC, Imlygic) |
| Immune system modulators |
A general term for agents that enhance the body’s immune response against cancer. The term immunomodulatory drug (IMID) is often used to describe a group of agents that stimulate the immune system. |
IMID:
Thalidomide (Thalomid)
Lenalidomide (Revlimid)
Pomalidomide (Pomalyst) |
Immunotherapy: Mechanism of Action
The activity of the immune system is regulated by a series of immune checkpoints.7-9 These proteins, located on the surface of immune cells, most notably the T-cells, have an important role in the control of immune cells function(s) and the immune response against malignant cells.8,9 These checkpoints serve as a series of on and off switches that regulate immune system, and specifically T-cell activity. On-switches (eg, CD28) serve to activate, whereas off switches (eg, CTLA-4) inhibit the immune cascade. Through a series of mechanisms immune checkpoints regulate T-cell activation and ultimately their activity, either by reducing CD28 co-stimulation (CTLA4) or by suppressing TCR/CD28 signaling (PD1).9
ICIs are a group of monoclonal antibodies that have been developed to target select immune checkpoints and inhibit the effect of that checkpoint on T-cell regulation.10 The first ICI marketed in the United States, ipilimumab, received Food and Drug Administration (FDA) approval for the treatment of metastatic and locally advanced melanoma in 2011.7 In the subsequent decade, new immune checkpoint targets and subsequent pharmacologic therapies have been identified, providing treatment options in an expanding number of tumor types.7 ICIs are now routinely used in the treatment of many cancers, and the role of these agents continues to expand in practice. Currently approved agents in the United States target 3 checkpoints, CTLA4, PD1, and PDL1 (Table 2).10 ICIs that target other immune checkpoints identified on T-cells, such as LAG3 or TIM3, are currently being evaluated in clinical trials.11 Because each checkpoint plays a unique role in the immune cascade, ICIs can be used alone or as part of dual therapy (eg, a combination of a CTLA-4 and a PD1 inhibitor). Additionally, ICIs are also now combined with other forms for anticancer therapy to optimize effectiveness in some cancers, including combination with chemotherapy in lung cancer or with multitargeted therapy in advanced renal cell carcinoma.12 The role of ICIs in combination with radiation or following definitive therapy is being explored to enhance treatment for diverse tumor types.
| Table 2. T Cell Immune Checkpoint Targets |
| Immune Checkpoint |
Immune Checkpoint Action |
Immune Checkpoint Inhibitors |
| CTLA-4 |
Modulates the immune response early at the time of T-cell activation by antigen presenting cells. CTLA4 binding to B7 ligands inhibits T cell activation. |
Ipilimumab (Yervoy) |
| PD-1 |
Limits the activity of T cells in peripheral tissues. The PD1 pathway impacts the T cell response at later stages of T cell activation. PD1 is upregulated on T cells after persistent antigen exposure, seen in cancer. |
Cemiplimab (Libtayo)
Nivolumab (Opdivo)
Pembrolizumab (Keytruda) |
| PD-L1 |
The ligand for PD1 that can be expressed by cancer cells and other hematopoietic and non-hematopoietic cells. |
Atezolizumab (Tecentriq)
Avelumab (Bavencio)
Durvalumab (Imfinzi) |
| LAG-364 |
Upregulation is required to control T cell activation and present the onset of autoimmunity. |
No commercially available agent marketed in the US. |
| TIM-365 |
Inhibition of immune response through induction of T cell apoptosis. |
No commercially available agent marketed in the US. |
Immune-Related Adverse Events: Pathophysiology, Prevalence & Incidence
Though ICIs are a relatively new treatment strategy compared with more historical anticancer approaches such as chemotherapy or radiotherapy, longitudinal data are emerging from patient cohorts treated with ICIs providing insights into acute, chronic, and late-effect toxicity profiles. ICIs have been described as “taking the breaks off the immune system.”13 Their therapeutic benefit, namely removing barriers to full immune system activity against tumor cells, is also the proposed mechanism of their unique toxicity profile. The resulting accelerated activity of immune cells can affect both malignant and healthy cells, resulting in irAEs across organ systems and tissue types.6 Several hypotheses exist related to the cause of these events and include T-cell activation against antigens that are present on both malignant and healthy tissue, increased levels of autoantibodies and activation of inflammatory cytokines, and complement-mediated inflammation resulting from anti-CTLA4 antibodies engaging with healthy tissues expressing CTLA4. 6 The reported incidence of ICI irAEs range from < 1% to 45% in published literature, 14 with some clinical trials reporting as many as 90% of participants receiving ICI experiencing an irAE.15 The incidence of irAEs appears to be dependent on patient-, disease- and treatment-related factors. Treatment-related factors include the target of the ICI agent, use as a single agent or in combination16 with other anti-cancer therapies, dose/treatment regimen, and/or duration of ICI therapy.17 The results of a systematic review of reported irAEs in clinical trials involving checkpoint inhibitors alone or in combination are summarized in Table 3. Although the data reported in Table 3 provide some understanding of irAE incidence with different treatment strategies, it is essential to consider both patient and disease-related factors.
| Table 3. irAE incidence from single-agent and combination therapies16 |
| Treatment |
PD(L)-1 |
CTLA-4 |
Dual ICI |
ICI + Chemotherapy |
| Grade 3 or greater |
14% |
34% |
55% |
46% |
| Adverse event requiring treatment withdrawal |
6% |
21% |
38% |
13% |
| Adverse event leading to death |
0.6% |
1.3% |
0.1% |
1.1% |
| *incidence and prevalence may vary greatly by treatment type, combination, and presentation |
It appears that some patients who respond to ICI therapy may have a prolonged response. In those individuals who are responsive to therapy, outcomes including complete response and long-term remission, have been observed. Early trials with ipilimumab in melanoma demonstrated that the risk of relapse over 5-year follow-up is estimated at less than 10%.18 As a result of individuals living longer, long-term and late effects may be observed years post-treatment completion.4 As questions regarding the optimal therapy duration of ICI continue to be explored, the life-sustaining effect of ICI therapies necessitates a proactive and long-term approach to irAE monitoring and management to be safely utilized in the care of cancer patients. As clinical trials are published, it is important to closely evaluate patient and disease-related factors that help us identify those individuals at highest risk for irAE.
Immune-Related Adverse Events by Organ System
irAEs can occur across all organ systems and in any tissue of the body. While the most common toxicities and associated symptoms observed with commercially available ICI include the skin (eg, rash and pruritis) and GI tract (eg, diarrhea), the prevalence of other toxicities may increase with the evolving application of these agents.4-6,16,19 Additionally, a common symptom observed in ICI clinical trials is fatigue, which can result from both direct and indirect effects of ICI. Here we summarize an overview of irAEs by organ system, as highlighted in Figure 1.
Figure 1. irAEs by System
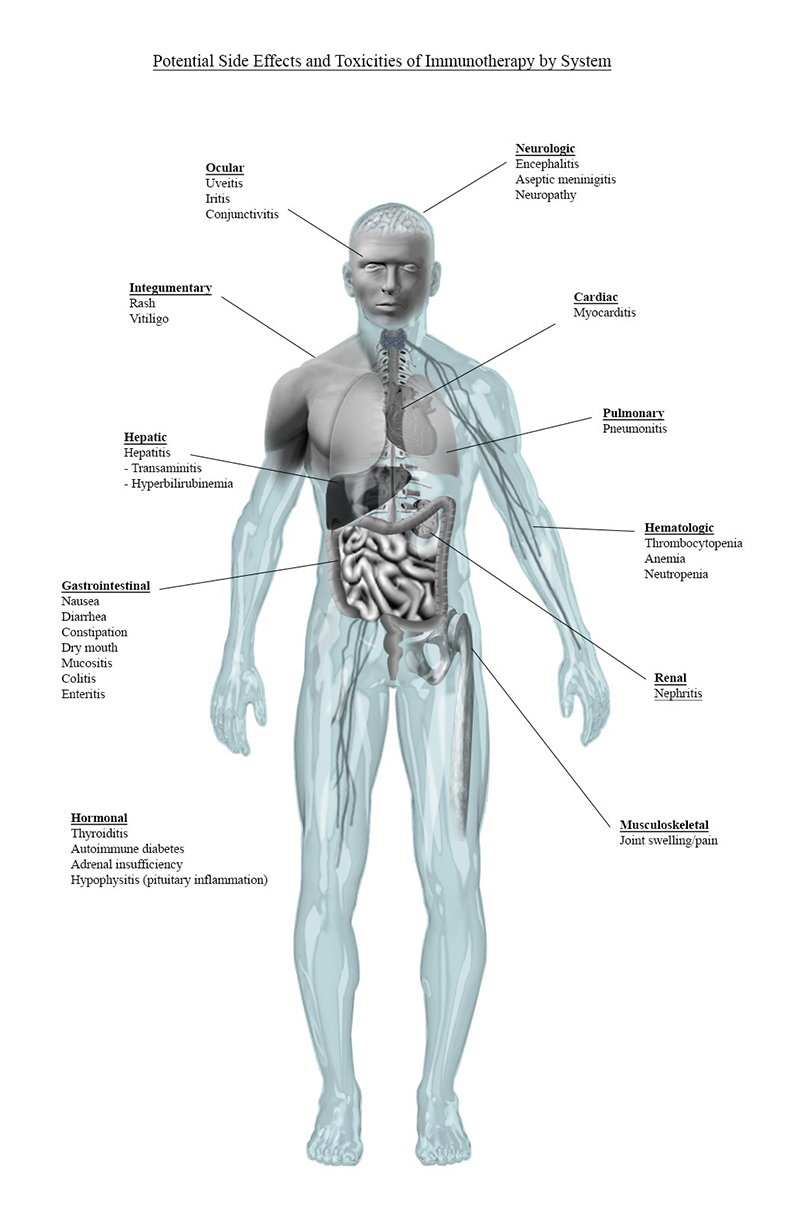
Review of Commonly Occurring irAEs
Some of the most commonly occurring symptoms of ICI-associated irAE are summarized in Table 4. While these manifestations are associated with more frequently observed irAEs, they do not occur in all patients receiving ICIs and are relatively unpredictable in onset of presentation. Some endocrine (ie, hypothyroidism, hyperthyroidism) and respiratory (ie, pneumonitis) irAEs appear to be more common with PD(L)-1 inhibitors compared to CTLA4 inhibitors, but the incidence may be related, in part, to the strategy of PD1/PDL1 inhibition. CTLA-4 inhibitors have been found to be more frequently associated with hypophysitis and colitis compared to PD1/PDL1 inhibitors. And ICI dual therapy is associated with increased incidence of colitis and hypothyroidism when compared with either treatment class alone. Importantly, the incidence of specific irAE is difficult to define by specific agent as the role of patient, disease, and treatment strategy also contributes to the inherent risk of irAE.
| Table 4. Frequently Observed Symptoms of ICI-Associated irAE16 |
Treatment
Overall incidence/(Grade 3-4) |
PD(L)-1
inhibitors |
CTLA-4
inhibitors |
Dual ICI |
ICI + Chemotherapy |
| Diarrhea |
11% |
36% (8%) |
44% (10%) |
------- |
| Rash |
10% |
23% |
41% (5%) |
------- |
| Pruritis |
15% |
25% |
34% (2%) |
------- |
| Fatigue |
21% |
25% |
------- |
------- |
| AST/ALT elevation |
------- |
------- |
------- |
31% (5%) |
Dermatologic irAE
Dermatologic irAEs are among the most prevalent, observed in 30% to 50% of individuals treated with ICIs, and more commonly associated with CTLA4 inhibitor therapy. 20 Dermatologic toxicities reported with ICI therapy may manifest as a simple maculopapular rash or as severe cutaneous adverse reactions (SCARS). Potential dermatologic manifestations include lichenoid reactions, acneiform rashes, vitiligo-like lesions, autoimmune skins disease, psoriasis, or sarcoidosis.20 The most common irAE cutaneous manifestation reported with ICI is a faint, erythematous maculopapular rash, often with pruritis, but patients and practitioners need to be aware that the dermatologic irAE vary greatly among patients.20-22 Identification, evaluation, and assessment of these toxicities are essential to direct appropriate treatment.21,23 A complete physical exam and assessment of both past dermatologic conditions and skin reactions, as well as current patient symptoms, is recommended for assessment of suspected dermatologic irAE. Close attention to evidence of more severe conditions like Stevens-Johnson Syndrome or Toxic Epidermal Necrosis is essential to mitigate the severity of symptoms and enable timely management.21,23 A dermatology consult is suggested for individuals with advanced grade or rare presentations.20-23 In general, management for Grade 1 and Grade 2 dermatologic toxicities typically includes the use of topical steroids and an oral antihistamine, with Grade 3 requiring a pause in treatment along with systemic corticosteroids.21,23 Grade 4 or higher dermatologic irAEs may require permanent discontinuation of the ICI.23
Endocrine irAE
Endocrine irAEs, which include a wide range of endocrine organ toxicity, have been reported to occur in up to 25% of patients receiving ICI therapy based on treatment type. Endocrine irAEs include hyper- or hypothyroidism (with hyperthyroidism proceeding hypothyroidisim), thyroiditis, adrenal insufficiency, diabetes, and hypophysitis, an inflammation of the pituitary gland.23,24 Many published guidelines now include recommendations for the routine monitoring of select laboratory tests (eg, TSH and cortisol) to facilitate early diagnosis of endocrine irAEs. Early diagnosis of endocrine irAEs expedite treatment of endocrinopathies before presentation of problematic symptoms. Presenting symptoms of irAE endocrinopathies vary by underlying endocrine dysfunction but may include general symptoms such as fatigue or weight gain; or organ specific symptoms such as constipation and cold intolerance (hypothyroidism); diarrhea, tachycardia, atrial fibrillation, heat intolerance, and weight loss (hyperthyroidism); weakness, nausea, vomiting, fever, and abdominal pain (glucocorticoid insufficiency); and fever, vomiting, electrolyte imbalance, hypotension, or coma (adrenal crisis).24 Evaluation of select laboratory tests may reveal hyponatremia, abnormal thyroid function, and low cortisol, ACTH, luteinizing hormone, follicle-stimulating hormone, prolactin, or testosterone depending on the organ involved.24 Diagnosis and evaluation of endocrinopathies is guided by assessment of laboratory abnormalities and a patient’s clinical presentation.24 Management of endocrinopathies often focuses on the treatment of organ specific damage (eg, replacement therapy in irAE hypothyroidism) but may include high-dose corticosteroids in some situations (eg, hypophysitis).23,24
Gastrointestinal irAE
Gastrointestinal (GI) irAE are observed during and following ICI therapy and may include diarrhea, colitis, and, less commonly, hepatitis. Diarrhea is the most common gastrointestinal irAE and has been reported to occur more commonly with CTLA4 inhibitors vs PD1/PDL1 inhibitors.25 The incidence of these toxicities increases with dual ICI therapy. Autoimmune-related inflammation of the gastrointestinal tract may result in frequent, watery stool, with the possibility for abdominal cramping and blood or mucus in the stool, in addition to inflammatory cell infiltration (eg, neutrophils), inflammatory changes, loss of vascularity, and ulcerations.25 It is important to obtain a thorough patient history, as many of the presenting symptoms of GI tract irAEs overlap with those occurring from other treatments or infection.25 The median onset to immune-related diarrhea in patients on anti–CTLA-4 therapy has been reported to be approximately 7 weeks as compared with approximately 6 to 18 weeks on an anti–PD-1 therapy (nivolumab and pembrolizumab) but may occur at any time during and following completion of therapy.25
Hepatic irAEs associated with ICI appear to be less common than diarrhea and colitis. Although the incidence appears to be < 5% in patients receiving single agent ICI, it is unclear what the incidence is with combination therapies or in patients with pre-existing liver risk factors (eg, alcohol use).26,27 The presentation of hepatitis frequently includes elevation of liver transaminases with or without hyperbilirubinemia. In one case series of 7 patients with hepatotoxicity associated with ICI therapy, liver injury shared some features seen with autoimmune hepatitis, but some differences were noted.26,27 Immune-mediated hepatotoxicity is a clinical diagnosis of exclusion, and it is important to carefully review a patient’s medication history and evaluate for other potential causes and/or potential contributing factors that range from drugs, alcohol, infection, thrombosis, and disease progression. Diagnosis primarily occurs via routine assessment of liver function tests (eg, elevated aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). If there is an increase in transaminases, an evaluation of bilirubin is indicated. Further evaluation may require imaging, such as abdominal CT.25 Immune-mediated hepatitis is most likely to be clinically detected approximately 8 to 12 weeks after ICI treatment initiation.25
Management strategies for gastrointestinal irAE are guided by their severity and manifestations, and they often require withholding the ICI and initiation of immunosuppressive and symptomatic management.23,25 While symptom support may permit treatment continuation at Grade 1, Grade 2 or higher symptoms may require holding or permanently discontinuing ICI therapy.23,25
Musculoskeletal irAE
Musculoskeletal or rheumatic irAEs were identified in 52 abstracts in a systematic review of the literature published in Arthritis Care & Research.28 The incidence of musculoskeletal irAE ranged in these reports, with arthralgias being seen in 1% to 43% and myalgias in 2% to 20% of patients included in trials. Manifestations of rheumatic irAEs vary greatly in patients and may include inflammatory arthritis, vasculitis, myositis, and lupus nephritis. Presentations may be inflammatory in nature and all new or changing inflammatory symptoms warrant evaluation through rheumatologic laboratory testing.28 The management strategy for these irAE are guided by the presentation and severity of symptoms, and patient-specific factors including comorbidities. Musculoskeletal irAE management may focus on pain management and may include analgesia, determined by patient-specific considerations. However, in the case of myositis, hospitalization for acute symptom management may be necessary due to the presence of severe limiting weakness that may require interventions such as plasmapheresis or IVIG therapy.23,28
Less Common and Unique irAE Profiles
Cardiovascular irAE
Though rare at this time, immune-related cardiotoxicities include pericarditis, left ventricular dysfunction, myocarditis, and cardiac arrest.29 Cases of fulminant myocarditis and myositis have recently been reported.29 Myocarditis is believed to be rare, occurring in < 1% of individuals, and can be observed as early as the first month of therapy, suggesting early onset of this irAE.29 Recommendations for monitoring for cardiovascular irAE with ICI include periodic laboratory screening (specifically for inflammatory markers ESR, CRP, and CK) in combination with electromyography and/or MRI. Clearly strategies for monitoring will evolve as we learn more about the irAE with ICI therapies, when used as single agents and when combined with other anticancer therapies. The management of patients with immune-related cardiotoxicity is guided by the presentation and grade of the adverse event. Treatment interruption should be evaluated on a case-by-case basis, with ICI treatment continuing in some patients with low-grade immune-related cardiotoxicities, and temporarily or permanently holding therapy may be required for others with higher grade toxicities.23
Hematologic irAE
Though rare, an analysis of the World Health Organization’s pharmacovigilance database identified 168 hematologic irAEs associated with ICI treatment.30 The scope of hematologic irAEs include immune thrombocytopenic purpura (ITP), hemolytic anemia (HA), hemophagocytic lymphohistiocytosis, aplastic anemia, and pure red cell aplasia. Monitoring for hematologic toxicities should include consistent evaluation of complete blood cell counts, with additional laboratory evaluation and assessments based on presenting symptoms.30 ICI therapy can sometimes be continued in the presence of Grade 1 hematologic toxicity; however, treatment should be held in the presence of a Grade 2 toxicity, as well as with the occurrence of Grade 1 acquired thrombotic thrombocytopenic purpura, aplastic anemia, or acquired hemophilia A.23 Management for many of the hematologic irAEs associated with ICI often includes immunosuppression (eg, corticosteroids)for early grade presentations with the addition of other therapeutic agents based on underlying diagnosis and presentation.23
Lung irAE
Pneumonitis, immune-mediate inflammation of the lining of the lung, can occur both during and following ICI treatment; the risk for which is increased on PD1 inhibitors or combination ICI therapy.14,19,31 Though not common, occurring in less than 10% of patients at any grade with single-agent ICI therapy, pulmonary irAEs can be fatal if not promptly treated.18,31 Presenting symptoms, including tachypnea, cough, and dyspnea, may mirror symptoms of disease (eg, lung cancer or pneumonia) or comorbidities (eg, chronic obstructive pulmonary disease); however, presence of pulmonary imaging resembling frosted glass is a potential differentiating sign.19,31 ICI treatment should be held in the presence of Grade 2 or higher pulmonary irAE and initiation of immunosuppression with corticosteroids is recommended, The addition of antibiotics in the presence of Grade 3 or greater toxicity maybe warranted.23 Baseline oxygen saturation measures and chest imaging should be obtained as comparison for status changes. Pulmonary function tests and arterial blood gases should be measured at baseline, particularly in the presence of hypoxia. Most patients treated for pneumonitis require symptom management in the acute care or emergency setting. Importantly, the critical need to recognize new or changing pulmonary symptoms in patients receiving ICI therapy mean that this less common toxicity is an important one to address with patients periodically throughout therapy.
Nervous System irAE
Neurologic irAEs have been reported in clinical trials of ICI at an incidence ranging from 0% to 27%. The presentation of neurologic irAE may include vague symptoms of headache, dizziness, confusion, aphasia, disorientation, agitation, and fatigue.32 More serious symptoms of encephalitis have also been reported. Central nervous system manifestations included cerebellar ataxia, dysarthria, bilateral internuclear ophthalmoplegia, and acute headaches.32 Additional rare presentations reported in case studies included encephalitis, cerebellitis, myelitis, and posterior reversible encephalopathy syndrome (PRES). While extremely rare to date, the occurrence of ICI-associated neurologic irAEs should be suspected in patients with any new neurologic symptoms and carefully monitored in individuals with any pre-existing neurologic conditions. In patients with pre-existing neurologic conditions, symptom exacerbation has been observed.32 ICIs may need to be held for new neurologic symptoms, even at low-grade presentation. A consult with a neurology specialist is recommended to inform the plan of care, which may include management with corticosteroids.23
Ocular irAE
Ocular toxicities are another rare toxicity occurring in less than 1% of individuals, and include peripheral ulcerative keratitis, uveitis, Vogt-Koyanagi-Harada syndrome, choroidal neovascularization and melanoma-associated retinopathy, thyroid-associated orbitopathy, and idiopathic orbital inflammation.33,34 Any change in vision should be a trigger to further evaluate for an ICI-related ocular adverse event. Treatment strategies are determined by the presentation and severity, with low-grade ocular irAE potentially managed with topical steroids. However, the potential impact of any damage to the eye and vision warrants consultation with an ophthalmologist for any known or suspected ocular irAE.23
Renal irAE
Renal toxicities of ICI therapies include acute kidney injury (AKI), acute interstitial nephritis, podocytopathy, and hyponatremia. Onset of renal irAE have been observed after approximately 2 to 3 months of treatment with CTLA-4 inhibitors and 3 to 10 months after treatment with PD1/PDL1 inhibitors.35 The incidences appears to vary based on the ICI agent administered and whether it was used alone or in combination. Importantly, the occurrence of AKI reported with ICI therapy has been estimated between 2.2% and 29%.35 Combination therapy with dual ICI therapy, including ipilimumab and nivolumab, was associated with a higher rate of occurrence (4.9%) than observed with single-agent treatment.35
The Evolution of irAE
The identification of irAEs continues to evolve as new ICIs are explore alone or in combination with ICIs and other anti-cancer therapies. It is important to note that immune-mediated inflammation can occur in any tissue or organ system of the body and that symptoms may manifest early in treatment, and months to years after completion of therapy. It is also important to recognize that even within systems that may be most frequently affected by irAEs (eg, skin and GI) there can be rare manifestations of toxicities.36 As such, effective management is necessary both within and beyond oncology care, and patients should be empowered to identify and communicate new or concerning signs and symptoms whether months or years post-treatment.
General Management Strategies for the Individual with irAE
The optimal management strategy for ICI irAEs presented in Figure 2 includes anticipation comprehensive assessment, early detection and identification, and treatment of the toxicity, followed by monitoring of treatment efficacy and consideration of ICI rechallenge.37-39 Treatment of ICI-related irAEs is guided by clinical presentation, severity, and patient-specific considerations.
Figure 2. Cycle of Assessment and Management of irAEs
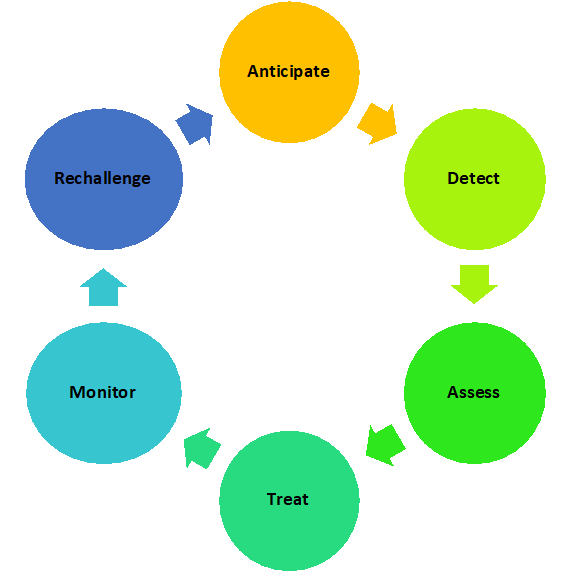
Assessment and Grading of irAE
A fundamental step in the optimal management of an individual with an ICI-associated irAE is early recognition of symptoms. The presentation of irAE varies greatly by organ system, and this is especially challenging with rare irAE. Further, irAEs are not always distinct or predictable in presentation, and signs and symptoms of irAEs may be easily attributed to disease-related symptoms, comorbidities, and/or concomitant medications. The presentation and symptoms of irAEs associated with ICIs may resemble toxicities associated with current or past chemotherapy, targeted therapy, and radiation therapy. It is easy to ascribe a cough due to ICI pneumonitis to lung cancer or to a comorbidity of chronic obstructive pulmonary disease. Recognizing an irAE symptom is compounded when ICIs are combined with other anticancer agents (eg, chemotherapy and/or targeted therapies) associated with similar manifestations of a toxicity, making it more difficult to identify the true cause of the adverse event. A new symptom may be the result of one or more irAE and/or comorbidities and/or concomitant disease; therefore, symptoms in a patient with current or past immunotherapy should be suspected to be associated with an irAE until ruled out. As the onset of symptoms for ICI-related irAEs are unpredictable and often delayed, it is essential to continually engage patients and caregivers to facilitate early detection of clinical symptoms that may herald an irAE.
Clinical practice guidelines have been developed by several professional organizations to guide practitioners in both the assessment and management of ICI-related irAEs (Table 5).23,40-42 These guidelines provide evidence and practice-based recommendations for practitioners regarding comprehensive toxicity evaluation, grading, and patient management for common and some more rare ICI-related irAEs. These guidelines are foundational resources for both oncology healthcare professionals, and members of the healthcare team that do not specialize in oncology but provide care for the individual with cancer. These guidelines should be readily available and familiar to primary care providers, urgent care centers, or emergency rooms, where individuals receiving ICI may present with new onset or progressing symptoms of ICI-related irAE.
Another foundational component of the optimal management of ICI-related irAEs is appropriate grading. The grading of treatment-related toxicities is established in the Common Terminology Criteria for Adverse Events (CTCAE). Grading is also described for more unique toxicities seen with ICIs in the published guidelines discussed.43 The landscape of immune-mediated injury to noncancer tissues is rapidly evolving; therefore, reviews and case reports of organ-specific irAEs are also a source for assessment and grading of irAE and provide insight in those areas not addressed in published guidelines. Clinical trials continue to provide insights into irAEs with ICIs in select populations to help practitioners best care for all patients.
General Approaches for Treatment of irAE
Interruption of ICI Therapy
Whether the irAE is common or rare, the approach to management of a patient with a suspected ICI-related irAE is determined by the affected tissue/organ, grade and manifestation of the toxicity, and patient-specific considerations. ICI therapy may be temporarily or permanently held, depending on these factors. Although holding planned ICI therapy is not routinely warranted for most Grade 1 irAE, it is recommended in some circumstances, including in the presence of some neurologic and cardiac toxicities.41 Importantly, patient-specific factors should help guide clinicians in the decision to hold or interrupt therapy. Delays in ICI treatment may be required for mild adverse events to better evaluate the symptoms and allow time for evaluation of treatment and symptom management. In select irAEs, such as hypothyroidism, delay in therapy is not recommended routinely even with higher grades of irAE. Whenever treatment is held, the duration of the delay in ICI therapy is guided by the irAE’s response to treatment, the downgrading of symptoms, and the overall impact of the irAE on the patient clinical status. ICI treatment is often resumed when the toxicity grade returns to 1 or less based on current guidelines.23,40-42
For more severe irAEs, discontinuation of the ICI is often required, although it may be temporary. The decision to re-challenge with ICI therapy is highly individualized for many irAEs.39 If ICI therapy is resumed, close follow up is required and most guidelines recommend collaboration with a specialist for system-specific management (eg, gastroenterologist for colitis). As understanding of the clinical presentation and management of irAEs continues to evolve, decisions about optimal management should be informed by the most recent versions of clinical practice guidelines as well as institutional practice parameters. These decisions should always be founded in the patient’s experience and unique presentation. Below are summarized some of the more common pharmacologic approaches to irAE management.
Immunosuppression in the Management of irAE
Immunosuppression is a key element in the treatment of many irAEs but is not the cornerstone of therapy for all irAEs. Endocrinopathies, such as hypothyroidism, are often managed with hormone replacement and do not warrant concomitant immunosuppression. The choice of immunosuppression agent, dose, route of administration, and duration of therapy continue to evolve in published guidelines and in clinical practice. For individuals receiving immunosuppression for irAE management, it is equally important to optimize the prevention and management of immunosuppression-associated complications. Immunosuppression is extremely beneficial in managing many irAEs, yet patient response to therapy and complications associated with immunosuppression are important considerations in individualization of treatment.
Immunosuppression: Corticosteroids
Corticosteroids are, at this time, a mainstay of treatment for most ICI-associated irAEs. The use of corticosteroids in the management of irAEs has not been shown to reduce the anticancer effect of ICIs. The dose, duration, and selection of corticosteroids is determined by the severity or grade of toxicity and patient-specific considerations including overall tolerability of corticosteroid therapy and impact on comorbidities. Published guidelines offer suggested dose ranges for corticosteroids, and individual patient factors should be considered when selecting an initial treatment regimen. High-dose corticosteroids (prednisone [PO] 1-2 mg/kg/d or methylprednisolone [IV] 1-2 mg/kg/d) may be chosen as an initial dose based on guidelines, but there is a large range in dose that must be contextualized in each individual’s unique presentation. Dose equivalency is presented in Table 6 to inform transitions in glucocorticoid use. The duration of corticosteroid therapy is determined by the patient’s response to treatment, and recommendations are provided in published guidelines for the suggested duration and tapering of corticosteroid therapy. Importantly, a recommendation to taper corticosteroids over 4 to 6 weeks should be guided by the patient’s response to therapy and observed relief of irAE-associated symptoms. Close monitoring of signs and symptoms of irAEs during each step of a corticosteroid taper is important to assure optimal management of irAEs. An increase in irAE symptoms may require a change in taper and result in a much longer and/or slower steroid taper to prevent irAE recurrences.
| Table 6: Approximate Relative Activities of Corticosteroids |
| Glucocorticoids |
Equivalent Dose (mg) |
| Dexamethasone |
0.75 |
| Methylprednisolone |
4 mg |
| Prednisone |
5 mg |
| Prednisolone |
5 mg |
| Hydrocortisone |
20 mg |
| Cortisone |
25 mg |
| This data is an approximate and does not reflect the differences of these agents in terms of mineralocorticoid activity.66 |
Monitoring during Corticosteroid Therapy
Corticosteroids are not without risk. Each patient placed on corticosteroids should be evaluated for individualized risk, including impact on comorbidities such as diabetes and/or hypertension. Medication management for comorbidities impacted by corticosteroid treatment should be prioritized, and close monitoring of the comorbidity should continue throughout the course and tapering of corticosteroids. Chronic medication dose adjustments should be considered prior to the start of corticosteroids, throughout the course of therapy, and importantly, at the time of corticosteroid taper. Engaging patients and caregivers in the monitoring of comorbidities (eg, home blood pressure and/or glucose monitoring) may be beneficial to optimize care.
Corticosteroids have many side effects, well known to oncology practitioners. It is important to consider the common toxicities of corticosteroids such as gastritis, and proactively evaluate the need for gastric acid lowering agents (eg, proton pump inhibitors or histamine 2 blockers). With the addition of any new medication, it is critical to assess the impact of the new medication provided to mitigate toxicities of corticosteroids (eg, proton pump inhibitors) on other concomitant medications. Because insomnia is observed with high-dose corticosteroids, optimizing the timing of steroid administration (eg, morning dosing for individuals who sleep at night) and proactively addressing sleep hygiene are important elements to care planning. Early intervention for steroid-associated insomnia may help optimize care and increase adherence to management strategies for irAEs.
As corticosteroid therapy may be required for an indeterminate or prolonged duration, medications for prophylaxis against pneumocystis pneumonia should be considered in patients receiving corticosteroids (eg, prednisone) at a dose of 20 mg (or equivalent) daily for 4 weeks or longer. Corticosteroid-associated osteoporosis is also a risk with long-term use of steroids. Vitamin D and calcium supplements, and integration of weight-bearing exercises, as appropriate for each individual patient, are strategies to mitigate this risk.
Patient education and counseling is critical for individuals receiving corticosteroids for irAE management. Written and oral instructions should be provided to patients and caregivers about planned therapy with steroids. Reinforcement that dosing and duration of therapy is determined by the response to therapy and coordinated by the oncology healthcare team is important as management of irAEs are guided by response. Patients should be counseled that they should not discontinue corticosteroids without instructions from the oncology healthcare team. For patients that are on long-term steroids, a medical alert bracelet and/or identification card may be helpful. In addition, instructions for increasing corticosteroid doses in situations of acute illness, stress, or medical procedures may be required for individuals on long-term corticosteroids due to adrenal suppression.
Immunosuppression: TNFα Inhibitors (eg,, infliximab)
Infliximab is recommended for the management of several steroid refractory irAEs. Infliximab, a monoclonal antibody targeting TNFα, binds and neutralizes soluble and membrane-bound TNFα resulting in the inhibition of pro-inflammatory cytokines, including IL1 and IL6. Current package labeling listed indications include Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, plaque psoriasis, and psoriatic arthritis. Black box warnings include reaction of latent tuberculosis, invasive fungal infection, and bacterial and viral infections due to opportunistic pathogens, as well as the risk for malignancy (lymphoma).44 Based on this warning, screening for tuberculosis (TB) should be considered prior to treatment.
There are still many questions regarding the optimal role of TNFα inhibitors, and infliximab specifically, in the management of irAEs. There is evidence that TNFα inhibition may have a different effect on tumor-specific immune response compared to corticosteroids, suggesting an opportunity to better define the role of these drugs in the management of irAEs as compared to other immunosuppressants.45 Timing and strategies for sequencing of immunosuppressive therapy continues to be investigated. Early intervention with TNFα inhibitors, such as infliximab, may help minimize the need for long-term corticosteroids and help limit corticosteroid-related toxicities. The best time to initiate TNFα inhibitor therapy is not clearly defined in current guidelines for most irAEs but will likely evolve as more is published about clinical experience with these agents. The optimal dosing and frequency for infliximab is also under investigation. Although a single dose of infliximab may be sufficient for management of an ICI-related irAE, additional doses may be required and are administered 2 to 6 weeks following initial dose. Infliximab is not recommended in patients with liver disease, including hepatitis from ICIs, due to the potential for associated liver injury. The role of TNFα blockade for the management of irAEs continues to evolve. A case report of concurrent TNFα blockade with concurrent ICI therapy to minimize irAEs provides initial insight into how to further optimize this therapeutic approach for the management of irAEs.46
Immunosuppresion: Vedolizumab
Vedolizumab is a monoclonal antibody that binds to the α4β7 integrin and blocks the interaction of the integrin with mucosal address in cell adhesion molecule-1 (MAdCaM-1), resulting in the inhibition of memory T cells across gastrointestinal tissue. The interaction of integrin with MAdCaM-1 appears to be important in the chronic inflammation seen with ulcerative colitis and Crohn’s disease. Current package labeling includes indications for Crohn’s disease and ulcerative colitis, as well as warnings for infusion-related reactions and hypersensitivity reactions, infections, progressive multifocal leukoencephalopathy, and liver injury.47 Based on these warnings, screening for tuberculosis (TB) should be considered prior to treatment. As with TNFα inhibitors, the role of vedolizumab continues to evolve especially in the management of gastrointestinal irAEs.
Supportive Care Strategies of irAE
As central as immunosuppressive strategies are for the management of individuals with ICI irAEs, it is important to also consider the overall supportive care needed for a patient with an irAE. For example, for patients experiencing ICI immune-related colitis, optimal care should include evaluation and management of symptoms (eg, diarrhea), symptom-related complications (eg, fluids and nutrition), and the impact of the irAE on comorbidities and overall health and clinical status. The treatment strategies outlined in published guidelines focus on interruption of ICI and immunosuppressive therapy and recommended pain management or supportive care consults as appropriate to manage symptom burden resulting from these treatment toxicities.23,40-42 In addition, a comprehensive review of all concomitant drug therapy should be completed, and dose modifications should be made based on the degree of organ dysfunction, if required.
Considerations in Specific Populations and irAEs
Patients Receiving ICIs as Part of Clinical Trials
A special consideration regarding ICI irAEs involves the individual receiving ICI therapy within the scope of a clinical trial. As the landscape of new therapies emerge, patients may be treated with agents that not only are commercially available but may also not yet have a formal name and is rather identified by a product number. If the ICI therapy is not currently marketed, information regarding the therapy is not readily available to the extended healthcare team and if a patient presents to their primary care provider or emergency department symptoms may not be recognized as an irAE. Communication with the oncology healthcare team is essential to optimize care. This is further confounded by patients who may be on blinded studies and therefore may not be aware if they are receiving treatment or placebo. For patients receiving ICI therapy with agents currently marketed as part of a clinical trial, the strategy for assessment and management of irAEs may be guided by the clinical trial protocol. Encouraging patients to communicate participation in clinical trials to their nononcology providers facilitates optimal care and is especially important when receiving a therapy associated with the broad spectrum of irAEs. Prompt consultation with the treating provider can also streamline care planning and the delivery of timely, safe care for the patient to support optimal outcomes.
ICIs continue to influence the treatment and outcomes of individuals with a number of cancers. Unfortunately, there are subgroups of patients that were infrequently enrolled in clinical trials because of disease status (eg, brain metastasis), comorbidities (eg, autoimmune disease), or concurrent medications (eg, corticosteroids). The rationale for some of the exclusion criteria was based, in part, on the concern for ICI safety in select populations. The concern, in practice, may be balanced by the need to learn more about the role of ICIs in these less studied clinical situations. Commentaries and case reports are increasingly being published addressing the use of ICIs in select populations that were excluded or limited in initial clinical trials and may serve as a valuable resource to the practitioner when considering therapy options in an individual patient.
Preexisting Autoimmune Disease and ICI
The use of ICIs in patients with preexisting autoimmune disease may result in severe inflammatory and autoimmune toxicities and compromise an individual’s ability to tolerate ICI therapy.48,49 As many of the clinical trials with ICIs specifically excluded history of autoimmune disease, published reports describing the experience of patients with known autoimmune disease receiving ICI therapy have only begun to emerge. A multicenter, retrospective cohort study of 112 patients was conducted to evaluate the safety and efficacy of ICIs in adult patients with preexisting autoimmune disease and cancer.49 The autoimmune diseases from this patient cohort included, but was not limited to, psoriasis, rheumatoid arthritis, and inflammatory bowel disease, and approximately 20% of patients were receiving immunosuppressive therapy at the time of ICI initiation. A flare of the preexisting autoimmune disease occurred in more than half the patients. Immunosuppression therapy was required in 43% and a permanent discontinuation of the ICI occurred in 21% of patients.49 Authors concluded that the use of ICIs in patients with preexisting autoimmune disease is safe but requires close monitoring.
Another consideration when weighing the potential risk vs the potential benefit of ICI therapy in an individual with a preexisting autoimmune disease includes assessment of current and past severity of the autoimmune disease.50 The risk of autoimmune exacerbation in an individual with a history of an autoimmune disease may be manageable; however, a discussion about risks and benefits with the patient and the entire healthcare team is important.
Because of the diversity in both cancer and auto-immune diagnoses, it is challenging to generalize the results of studies to the unique condition of an individual patient with such comorbidities. Published studies that report the experience of the patients with preexisting autoimmune diseases that received ICI may not reflect the risk of a select autoimmune disease. Using best available evidence now and referencing expanding literature with unique case studies may serve to bridge the gap remaining from the initial clinical trials.
Collaborative care planning with the provider managing the autoimmune diagnosis is important prior to the initiation of therapy to promote optimal outcomes for all underlying conditions. Close monitoring of the patient by both the oncology-focused and multidisciplinary healthcare team is required to identify any autoimmune disease flares and to facilitate early intervention. In addition, larger studies are needed to better identify the risks of ICI therapy in individuals with specific autoimmune diseases of varying severity.
Patients following transplantation
Safety and efficacy data are limited for the use of ICIs in patients who have undergone solid organ or hematopoietic stem cell transplantation, as they were also excluded from most ICI clinical trials. Patients that undergo transplantation require fine-tuned modulation of immunosuppression to maintain allograft tolerance and prevent organ rejection. Further, ICI therapy before and following allogeneic stem cell transplantation is believed to be associated with increase graft-vs-host disease, which should be carefully reviewed in treatment planning and subsequent monitoring.51 ICI therapy in patients following transplantation requires careful consideration of potential efficacy for the treatment of cancer weighed against the potential risk associated with impact of ICI therapy on the transplanted organ. Considerations may include the risk of organ loss in solid organ transplantation or occurrence of graft vs host in a patient following allogeneic stem cell transplantation. At this time, several prospective trials and case reports provide some insight in the use of ICIs in patients after solid organ and hematopoietic stem cell transplantation.52,53
Females of Reproductive Potential
As with many other anticancer therapies, consideration regarding the impact of ICI therapy on pregnancy and fertility should be considered. This is especially important for women of reproductive potential due to the risk of using ICIs during pregnancy. Regulatory T cells are potent mediators of self-tolerance and essential for suppression of immune response in pregnancy.54 The PD1/PD-L1 interaction is involved in maintaining fetal tolerance, and ultimately pregnancy, through induction of maternal immune tolerance to fetal tissues. Inhibition of PD1/PD-L1 could decrease fetal tolerance and result in loss of pregnancy. Animal studies have demonstrated that PD1/PD-L1 inhibition increases the risk of spontaneous abortions, although the impact of CTLA4 inhibition is less clear.52 The package labeling for all commercially available ICIs in the United States recommends verification of pregnancy status in females of reproductive potential prior to initiation of ICI therapy. Each marketed ICI agent provides specific recommendations within package labeling regarding counseling guidance for females of reproductive potential. This includes the recommendation for effective contraception during ICI therapy and following final dose of ICI therapy as outlined in Table 7. Additionally, package labeling for each product provides recommendations regarding breastfeeding during and following therapy. These recommendations are based on the potential for adverse effects in breastfed children in woman receiving ICI therapy.
| Table 7: Recommendations for Contraception and Lactation (per package labeling) |
| Agent |
Duration for Contraception (females of reproductive potential) |
Breastfeeding |
| Atezolizumab1 |
During treatment and for at least 5 months following final dose. |
Do not breastfeed during therapy and for at least 5 months after the final dose. |
| Avelumab2 |
During treatment and for at least 1 month following final dose. |
Do not breastfeed during therapy and for at least 1 month after the final dose. |
| Cemiplimab3 |
During treatment and for at least 4 months following final dose. |
Do not breastfeed during therapy and for at least 4 month after the final dose. |
| Durvalumab4 |
During treatment and for at least 3 months following final dose. |
Do not breastfeed during therapy and for at least 3 months after the final dose. |
| Ipilimumab5 |
During treatment and for at least 3 months following final dose. |
Do not breastfeed during therapy and for at least 3 months after the final dose. |
| Nivolumab6 |
During treatment and for at least 5 months following final dose. |
Do not breastfeed during therapy and for at least 5 months after the final dose. |
| Pembrolizumab7 |
During treatment and for at least 4 months following final dose. |
Do not breastfeed during therapy and for at least 4 months after the final dose. |
1Tecentriq [package insert]. South San Francisco, CA: Genentech, Inc.; 2020.
2Bavencio [package insert]. Rockland, MA: EMD Serono, Inc.; 2020.
3Libtayo [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc./sanofi-aventis; 2020.
4Imfinzi [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020.
5Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2020.
6Opdivo [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2020.
7Keytruda [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2020. |
Management Tools
Clinical Practice Guidelines
As discussed, there are several evidence-based guidelines available for the management of irAEs.23,40-42 Each provides a unique perspective informed by both clinical trial outcomes and practice-based evidence and are outlined in Table 5. The selection of which guideline to use may be based on regional, institutional, or personal preference and may involve the use of one or more guidelines to inform best practice. The frequency of the updating of a guideline maybe an important consideration when selecting a resource to ensure clinical practice is guided by the most current available evidence.
Mobile Applications for irAE Grading
Recently mobile applications55 have emerged to assist clinicians in grading irAEs, available for both iOS56 and Android57 mobile devices. The IO Tox Management Application is based on the ASCO guidelines for the management of ICI-related toxicities and the National Cancer Institute (NCI) guidelines for the grading of adverse events (V4). The app optimizes patient care by providing standardized, evidence-based algorithms to accurately grade and manage suspected irAEs. It is broadly available at no charge and can be used on both institutionally issued and personal devices to support adoption across practice settings where irAEs may be managed.
Interprofessional Approach to irAE Management
Because the occurrence of irAEs is organ specific and requires unique monitoring and management based on the organ site or presentation, it is strongly recommended that an interprofessional approach to therapy, with early integration of specialists (eg, gastroenterology, dermatology) for each organ system affected, be standardized in practice.58 While the guidelines provide a general standardized approach to care, ultimately these toxicities are best managed by a collaborative team including both oncology providers and disease specialists if possible. If a specialist is not available in your clinical setting, consider strategies such as a telehealth consult, both with the patient, or with community-based providers, to guide best practice.59 The importance of interprofessional management is highlighted as a recommendation in several of the irAE clinical practice guidelines.
The Unique Role of Nurses and Pharmacists in irAE Management
Nurses and pharmacists are uniquely positioned to support the monitoring, identification, and management of irAEs. Patient and caregiver education prior to ICI therapy, and throughout treatment, is essential and is a shared role for the entire oncology healthcare team. In a clinical practice, that role may be designated to a specific member of the oncology healthcare team, but these concepts should be reinforced consistently by each team member throughout treatment. Recognition, and subsequent assessment of patient symptoms, is routinely done by all members of the oncology healthcare team and should be coordinated to assure early identification of a new or changing symptom. Additionally, comprehensive review of drug therapy for a patient receiving ICI should be conducted at each treatment visit to identify any new or changing prescriptions and evaluate their potential effect on ICI treatment or toxicities. A review of over-the-counter medications may help identify a patient who has initiated self-treatment for an early sign of an irAE and has not recognized the importance of the new symptom. In addition, nurses and pharmacists are essential in developing institutional resources to support optimal care for patients receiving ICIs. Developing treatment order sets for electronic health record systems that incorporate routine evaluation of appropriate signs, symptoms, and laboratory tests help to trigger appropriate assessments.
Managing irAEs Across Practice Settings
Because of the longitudinal and late effects of checkpoint inhibitor therapy, individuals with a history of ICI treatment are likely to be managed in nononcology settings, including primary and emergency care settings.60,61 As such, it is important that both patients and providers are counseled about, and have available, resources to guide the management of irAEs. This is particularly important in the nononcology care setting. Utilization of clinical practice guidelines and the mobile toxicity applications mentioned earlier can support evidence-based management both within and beyond the oncology setting. This also applies to other members of a patient’s healthcare team, including primary care providers, dentists, ophthalmologists, and others providing care beyond the typical cancer care setting, to ensure that all members of the patient care team take into consideration ICI treatment in their own plan of care.
Patient-Centered Approach to irAE Monitoring and Management
The use of ICI is rapidly evolving, and the identification and management of irAE are central to the effective use of ICI therapy. Patients are essential to the optimization of care and should be able to communicate about their treatment, particularly with healthcare providers beyond the oncology team. An important focus of patient counseling should be the critical importance of reporting any new symptoms or changes in clinical status early and throughout and following completion of ICI therapy. Delays in symptom reporting, whether due to perception of nonseverity, concern about hospitalization, or lack of awareness, can contribute to presentation at advanced toxicity grades and delay in timely therapy. Reassuring patients and caregivers that the development of an irAE does not automatically require discontinuation of ICI therapy may help to proactively alleviate concerns about reporting clinical changes and facilitate open communication about new symptoms.
Given the potential for irAE management beyond the oncology setting, it is important to empower patients with the resources and education needed to inform new or nononcology providers about their care. This can be achieved through the use of resources, such as a pocket card with documentation of past and present immunotherapy-based treatment.62 Published management guidelines23,40-42 for ICI-related irAE stress the need for communication and care coordination between patients and their oncology and nononcology providers. While electronic health records (EHR) may provide access to providers within a designated healthcare system, care provided to patients beyond this system may not be integrated into the EHR.
Providing evidence-based resources, whether from the clinical institution, federal agencies, professional, or patient-facing organizations is important and should be aligned to both the health literacy and informational preferences of the patient. Recent tools have focused on providing patient-centered educational resources to reduce the complexities of ICI treatment and irAE management.63 Examples of digital and printable materials for patients are included in Table 8.
| Table 8. Patient Educational Resources |
| Patient Education |
| Organization |
Content |
Access |
| National Cancer Institute |
Immunotherapy to Treat Cancer |
https://www.cancer.gov/about-cancer/treatment/types/immunotherapy#what-are-the-side-effects-of-immunotherapy |
| American Cancer Society |
Immune Checkpoint Inhibitors and their Side Effects |
https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html |
| European Society of Medical Oncology |
Patient Guide on Immunotherapy-Related Side Effects and Their Management |
https://www.esmo.org/for-patients/patient-guides/immunotherapy-side-effects |
| Society for the Immunotherapy of Cancer |
SITC connected Patient Resources |
https://www.sitcancer.org/connectedold/p/patient |
| National Comprehensive Cancer Network |
Understanding Immunotherapy Side Effects |
https://www.nccn.org/images/pdf/Immunotherapy_Infographic.pdf |
Conclusion
The role of the immune response in cancer has become an important focus in the understanding and the management of cancer. ICIs, as single agents and in combination with other anticancer therapies, are now commercially approved for first-line and later treatment and ongoing clinical trials are exploring new targets, agents and combinations for diverse malignancies. The success of these agents requires optimizing the care of patients who receive ICI, including the management of treatment-related complications, specifically irAEs. The anticipation, detection, evaluation, and treatment of irAEs are a fundamental component of care for patients during and following ICI treatment. Because of the potential for long-term and late effects of ICIs, even after the discontinuation of treatment, it is imperative that patients and their caregivers are engaged with and provided evidence-based education and resources to be able to communicate their treatment history and any new or worsening symptoms to healthcare providers both with and beyond the oncology setting. Resources such as clinical practice guidelines, patient and provider facing education, and ICI-focused tools, like pocket cards, can support both patients and providers in ensuring safe, time, and effective care for individuals with current or prior ICI-based therapy. Nurses and pharmacists both within and beyond the oncology setting are pivotal to the engagement, education, and assessment of individuals eligible for or treated with ICIs, achieved through patient-provider partnership focused on evidence-based and patient-centered care.
Abbreviations:
CTLA4 – cytotoxic T-lymphocyte associated protein 4
ICI – immune checkpoint inhibitor(s)
irAE – immune-related adverse event
LAG3 – lymphocyte-activation gene 3
MAdCaM-1 - mucosal addressin cell adhesion molecule-1
PD1 – programmed cell death protein 1
PDL1 – programmed cell death ligand 1
TNFα – tumor necrosis factor alpha
TIM3 – T cell immunoglobulin and mucin domain-3
References
- Nakajima H, Nakatsura T. Towards the era of immune checkpoint inhibitors and personalized cancer immunotherapy. Immunological Medicine. 2020:1-6. doi:10.1080/25785826.2020.1785654.
- Brassil KJ, Ginex PK. History of immunotherapy. In: Walker S, Dunphy EP, eds. Guide to Cancer Immunotherapy. Pittsburgh, PA: Oncology Nursing Society; 2018:1-18.
- Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Sem Oncol Nurs. 2019;35(5):150923. doi:10.1016/j.soncn.2019.08.002.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Prim. 2020;6(1). doi:10.1038/s41572-020-0160-6.
- Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr Oncol. 2018;25(5). doi:10.3747/co.25.4235.
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi:10.1056/nejmra170348.
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Comm. 2020;11(1). doi:10.1038/s41467-020-17670-y.
- Dyck L, Mills KH. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47(5):765-779. doi:10.1002/eji.201646875.
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8. doi:10.3389/fonc.2018.00086.
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Intern Immunopharmacol. 2018;62:29-39. doi:10.1016/j.intimp.2018.06.001.
- Qin S, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1). doi:10.1186/s12943-019-1091-2.
- Wu Y, Shi H, Jiang M, et al. The clinical value of combination of immune checkpoint inhibitors in cancer patients: A meta-analysis of efficacy and safety. Int J Cancer. 2017;141(12):2562-2570. doi:10.1002/ijc.31012.
- Weigmann K. Releasing the brakes to fight cancer. EMBO Rep. 2016;17(9):1257-1260. doi:10.15252/embr.201643038.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563-580. doi:10.1038/s41571-019-0218-0.
- Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi:10.1056/nejmoa1003466.
- Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int Immunopharm. 2018;63:292-298. doi: 10.1016/j.intimp.2018.08.014.
- Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2017;36(4):638-646. doi:10.1007/s10637-017-0534-0.
- Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi:10.1056/nejmoa1003466.
- Liu Y-H, Zang X-Y, Wang J-C, Huang S-S, Xu J, Zhang P. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed Pharmacother. 2019;120:109437. doi:10.1016/j.biopha.2019.109437.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Dermatol. 2018;19(3):345-361.
- Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management. J Cutan Med Surg. 2020;1203475420943260. doi: 10.1177/1203475420943260.
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93(1):123-132.
- Brahmer JR, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guidelines. J Clin Oncol. 2018;36:1-55.
- Illouz F, Briet C, Cloix L, et al. Endocrine toxicity of immune checkpoint inhibitors: essential crosstalk between endocrinologists and oncologists. Cancer Med. 2017;6(8):1923-1929. doi:10.1002/cam4.1145.
- Cramer P, Bresalier RS. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep. 2017;19(1):3. doi:10.1007/s11894-017-0540-6.
- Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;(31):965-973.
- Peeraphatdit T, Wang J, Odenwald, et al. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315-329.
- Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken). 2017;69(11):1751-1763. doi:10.1002/acr.2317.
- Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2(4):e000247. doi:10.1136/esmoopen-2017-000247.
- Davis EJ, Salem JE, Young A, et al. Hematologic complications of immune checkpoint ihibitors. Oncologist. 2019;24(5):584-588. doi:10.1634/theoncologist.2018-0574.
- Su Q, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108. doi: 10.3389/fimmu.2019.00108.
- Möhn N, Beutel G, Gutzmer R, Ivanyi P, Satzger I, Skripuletz T. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy-review of the literature and future outlook. J Clin Med. 2019;8(11):1777. doi:10.3390/jcm8111777.
- Parikh RA, Chaon BC, Berkenstock MK. Ocular complications of checkpoint inhibitors and immunotherapeutic agents: a case series. Ocul Immunol Inflamm. 2020;1-6. doi:10.1080/09273948.2020.1766082.
- Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288-294. doi:10.1097/CCO.0000000000000296.
- Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45(2):160-169. doi:10.1159/000455014.
- Schoenfeld SR, Aronow ME, Leaf RK, Dougan M, Reynolds KL. Diagnosis and management of rare immune-related adverse events. Oncologist. 2020 Jan;25(1):6-14. doi: 10.1634/theoncologist.2019-0083.
- Champiat S, Lambote O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27(4):559-574.
- Helissey C, Vicier c, Champiat S. The development of immunotherapy in older adults: new Treatments, new toxicities? J Geriatric Oncol. 2016;7:325-333.
- Haanen J, et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer. 2020;8(1):e000604. doi: 10.1136/jitc-2020-000604.
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):119-142.
- Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020.
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95.
- Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6(2):154-160. doi:10.4103/apjon.apjon_3_19.
- Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc. 2020.
- Draghi A, Borch TH, Radic HD, et al. Differential effects of corticosteroids and anti-TNF on tumor specific immune responses: implications for the management of irAEs. Int J Cancer. 2019;145:1408-1413.
- Badran YR, Cohen JV, Brastianos P, et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse effects. J Immunother Cancer. 2019;7(1):226.
- Vedolizumab [package insert]. Deerfield, IL: Takeda Pharmaceuticals America; 2020.
- Coureau M, Meert AP, Berghmans T, Grigoriu B. Efficacy and toxicity of immune -checkpoint inhibitors in patients with preexisting autoimmune disorders. Front Med (Lausanne). 2020;7:137. doi: 10.3389/fmed.2020.00137.
- Tison A, Quere G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100-2111.
- Remon J, Hendriks L, Aspeslagh S, Besse B. Is there room for immune checkpoint inhibitors in patients who have NSCLC with autoimmune diseases? J Thorac Oncol. 2019;14(10):1701-1703.
- Ijaz A, et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transpl. 2019;25(1):94-99. doi: 10.1016/j.bbmt.2018.08.028.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106.
- Jorgensen N, et al. The tolerogenic function of regulatory T cells in pregnancy and cancer. Fron Immunol. 2019(10):1-21.
- Wild D. New App helps clinicians manage checkpoint inhibitor AEs; Clin Onc News. Retrieved from https://www.clinicaloncology.com/Current-Practice/Article/07-20/New-App-Helps-Clinicians-Manage-Checkpoint-Inhibitor-AEs-/59324.
- The Ronin Project, LLC. IO Tox Management (version 1.0.3) [Apple OS application]. Retrieved from https://apps.apple.com/us/app/io-tox-management/id1514006592, Published 2020; Accessed October 12, 2020.
- The Ronin Project, LLC. IO Tox Management (version 1.6) [Android OS application]. Retrieved from https://play.google.com/store/apps/details?id=com.projectronin.iotoxman, Published 2020; Accessed August 15, 2020.
- Kottschade L, Brys A, Peikert T, et al. A multidisciplinary approach to toxicity management of modern immune checkpoint inhibitors in cancer therapy. Melanoma Res. 2016;26(5):469-480. https://doi.org/10.1097/CMR.0000000000000273.
- Cole S, Zibelman M, Bertino E, Yucebay F, Reynolds K. Managing immuno-oncology toxicity: top 10 innovative institutional solutions. Am Soc Clin Oncol Educ Book. 2019 Jan;39:96-104. doi: 10.1200/EDBK_100018.
- Daniels GA, Guerrera AD, Katz D, Viets-Upchurch J. Challenge of immune-mediated adverse reactions in the emergency department. Emerg Med J. 2019;36(6):369-377. doi:10.1136/emermed-2018-208206.
- Simmons D, Lang E. The most recent oncologic emergency: what emergency physicians need to know about the potential complications of immune checkpoint inhibitors. Cureus. 2017. doi:10.7759/cureus.1774.
- Oncology Nursing Society. Immunotherapy Wallet Cards. Retrieved October 1, 2020 from https://www.ons.org/clinical-practice-resources/immunotherapy-patient-wallet-card
- Yanez B, Bouchard LC, Cella D, et al. Patient-centered engagement and symptom/toxicity monitoring in the new era of tumor next-generation sequencing and immunotherapy: The OncoTool and OncoPRO platforms. Cancer. 2019;125(14):2338-2344. doi:10.1002/cncr.32030.
- Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80-96.
- Friedlaender A, Addeo A, Banna G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019;4:e000497.
- Beale JM, Block JH. Wilson and Grivold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry. 12th Philadelphia, PA: Lippincott Williams & Wilkins; 2011:853-858.