
Expired activity
Please go to the PowerPak
homepage and select a course.
GLP-1 Receptor Agonists for the Management of Type 2 Diabetes: Pharmacist Focus on the Evolving Treatment Landscape
What do we Currently Know about the Cardiovascular Benefits of GLP-1 RAs? A Primer for Pharmacists (Part 2)
CARDIOVASCULAR OUTCOME TRIALS IN DIABETES
In late 2008, the United States (U.S.) Food and Drug Administration (FDA) published a Guidance for Industry that contained recommendations to be immediately adopted by pharmaceutical companies seeking approval for marketing new medications for the treatment of type 2 diabetes mellitus (T2DM).1 This abrupt change came in response to raised concerns from certain investigations that documented an unexpected increase in cardiovascular (CV) risks for patients with T2DM randomized to active treatment arms of clinical trials.2,3 The guidance outlines requirements for manufacturers that aim to conduct randomized trials specifically designed to evaluate CV safety of new medications prior to submission of New Drug Applications (NDAs).
Since the publication of the Guidance for Industry, more than 20 cardiovascular outcome trials (CVOTs) have been conducted to document CV safety, and sometimes CV benefit, over conventional care alone (Figure 1). To date, all CVOTs for dipeptidyl peptidase 4 (DPP-4) inhibitors,4-7 glucagon-like peptide-1 receptor agonists (GLP-1 RAs),8-13 and sodium-glucose transport protein 2 (SGLT-2) inhibitors14-16 have documented a lack of increased CV risk (non-inferiority) with the investigational product in patients with T2DM compared to placebo. Further, several CVOTS evaluating GLP-1 RAs8,9,13 and SGLT2 inhibitors14,15 have documented significantly reduced CV event rates compared to placebo.
| Figure 1. Timeline for Cardiovascular Outcome Trials Since Publication of the Guidance for Industry |
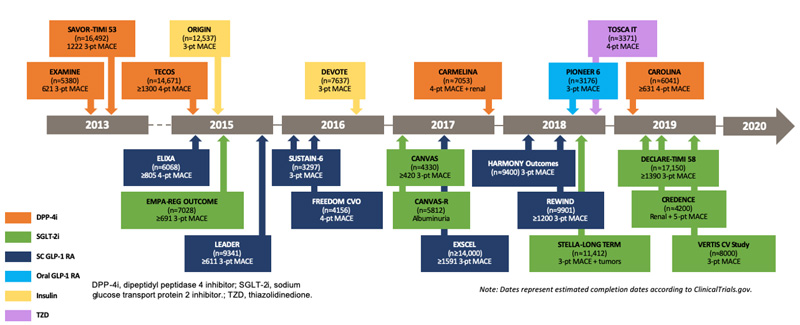
click here for a larger image |
| DPP-4i, dipeptidyl peptidase 4 inhibitor; MACE, major adverse cardiovascular event; SC, subcutaneous; SGLT-2i, sodium glucose transport protein 2 inhibitor; TZD, thiazolidinedione. |
HISTORICAL PERSPECTIVE LEADING TO THE GUIDANCE FOR INDUSTRY
The University Group Diabetes Program (UGDP)17 was among the first to shed light on CV implications of therapies used to treat T2DM (Figure 2).2,17-22 During this investigation, increased CV mortality was found in patients assigned to the first-generation sulfonylurea tolbutamide; this result has not been repeated with second-generation sulfonylureas. Decades later, the United Kingdom Prospective Diabetes Study (UKPDS)18 documented significant microvascular benefit for patients with new-onset T2DM randomized to intensive glycemic control with insulin or a sulfonylurea compared to patients using conventional therapy with diet. At the end of the study, the intensive-control group achieved a glycated hemoglobin level (A1C) of 7.0%; the standard of care group achieved an A1C of 7.9%. The intensive-therapy group also achieved a 25% risk reduction for microvascular complications (95% CI, 7%-40%; p=0.0099). At that time, no impact on macrovascular risk was noted; however, during the open-label follow-up, delayed risk reductions of 15% for myocardial infarction (p=0.01) and 13% for all-cause mortality (p=0.007) were observed.19 Similar findings were validated by the Diabetes Control and Complications Trial (DCCT),20 which evaluated intensive blood glucose control using continuous subcutaneous insulin infusion or 3 or more daily insulin injections compared to conventional therapy with 1 or 2 daily insulin injections in patients with type 1 diabetes. These 2 studies fortified the concept that lowering blood glucose closer to the euglycemic range prevents or delays development of microvascular complications such as retinopathy, nephropathy, and neuropathy.18,20
The Action to Control Cardiovascular Risk in Diabetes (ACCORD),2 Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),21 and Veterans Affairs Diabetes Trial (VADT)22 were later conducted under the auspice that it is never too late to intensify glucose control. Unlike the UKPDS, enrolled patients in these studies had long-standing and chronically uncontrolled T2DM. While ADVANCE and VADT documented neutral impacts of intensive glycemic reductions, ACCORD demonstrated an increase in the risks of all-cause death (HR, 1.22; 95% CI, 1.01-1.46; p=0.04) and CV mortality (HR, 1.35; 95% CI, 1.04-1.76; p=0.02). Interestingly, the thiazolidinedione rosiglitazone was used more often (91.2%) in the intensive-therapy arm than in the standard-therapy arm (57.5%). These 3 studies blunted the argument to universally intensify glycemic control in all patients: rather, they highlighted the need for individualized glycemic targets.
| Figure 2. Timeline for Historical Diabetes Outcome Trials2,17-22 |
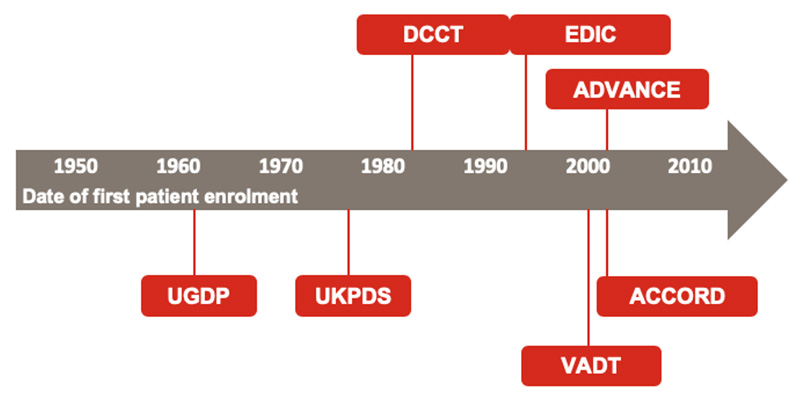 |
| ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; DCCT, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; UGDP, University Group Diabetes Program; UKPDS, United Kingdom Prospective Diabetes Study; VADT, Veterans Affairs Diabetes Trial. |
Further outcome data emerged for the thiazolidinedione rosiglitazone, which, again, highlighted the need for safety-focused CVOTs. The RECORD study demonstrated a 2-fold greater risk of the incidence of heart failure (HF) with the addition of rosiglitazone in patients with T2DM compared to placebo (HR, 2.1; 95% CI, 1.35-3.27).23 A combination of these data with a meta-analysis3 reviewing rosiglitazone use, which showed a 43% increase in the rate of myocardial infarction (p=0.003) and a 64% nonsignificant increased risk of CV death (p=0.006) in patients using the drug, ultimately compelled the FDA to take immediate action toward changing the standard requirements for documenting safety of new medications seeking approval for T2DM treatment.
Prior to these unexpected results, which stimulated the development and release of the FDA’s Guidance for Industry,1 CVOTs for medications awaiting FDA approval for treating T2DM were founded on documented surrogate markers for efficacy such as A1C or blood glucose decline. No documentation related to hard outcomes, such as macrovascular or microvascular complications, were needed to gain FDA approval for marketing in the U.S., despite the known 2- to 4-fold greater risk for experiencing CV events in individuals living with diabetes.24 However, since 2008, all non-insulin medications requesting FDA approval for the treatment of T2DM are now expected to prove CV safety alongside glycemic improvements. Because type 1 diabetes results from an absolute deficiency of endogenous insulin production, exogenous insulin therapy is a requirement for sustaining life and is, therefore, exempt from the heightened expectations of the Guidance for Industry.1 Further, those living with T2DM have a large array of therapeutic options for controlling blood glucose beyond exogenous insulin, and more opportunities exist for evaluating the CV safety of therapies in this patient population.
The Guidance for Industry suggests that pharmaceutical manufacturers complete studies evaluating CV-specific outcomes in patient populations representing those commonly treated for T2DM before submission of an NDA. Past studies often excluded those with advanced age, CV risk, long-standing diabetes, or renal dysfunction, despite these populations being commonly treated for T2DM in daily practice. Thus, the Guidance for Industry specifically directs the inclusion of high-risk populations in CVOTs: those at high CV risk, those with advanced disease, elderly patients, and those with some degree of renal dysfunction.1
Contrary to previous studies, there is no focus on improving glycemic control in CVOTs. In fact, CVOTs are designed to maintain glycemic equipoise, allowing for therapeutic augmentation to achieve desired blood sugar outcomes between the investigational therapy and placebo. This allows for any CV difference noted to be best attributed to the study medication rather than to a difference in A1C. CVOTs specifically evaluate the incidence of major adverse cardiovascular events (MACE), which includes CV mortality, non-fatal myocardial infarction, and non-fatal stroke, commonly known as “3-point MACE.” Hospitalization for acute coronary syndrome, urgent revascularization procedures, and other possible endpoints such as hospitalization for angina or HF can also be incorporated into the CV outcome endpoints of interest. Secondary outcomes commonly include the individual components of the primary outcomes, all-cause mortality, and time to first occurrence of hospitalization for HF, angina, or acute coronary syndrome. Other secondary or exploratory outcomes include an expanded composite endpoint, which adds death from any cause or coronary revascularization to the 3-point MACE. At times, other endpoints focus on microvascular outcomes, such as individual or composite renal or retinal endpoints or new or worsening nephropathy. Each of the primary composite and other endpoints are prospectively adjudicated in a blinded fashion by an independent CV endpoint committee.
Phase 3 studies must prove that the investigative medication does not increase the CV incident risk rate by more than 80%, with a margin of non-inferiority (or delta [d]) of 1.8. This means that the upper bound of the 2-sided 95% confidence interval must fall below 1.8 of the estimated risk ratio to prove non-inferiority. New medications whose CVOTs result in the upper bound of the 95% confidence interval lying above 1.8 will not be approved for marketing. Conversely, those falling below 1.8 can be approved, pending that the overall risk-benefit analysis supports approval. When the upper bound of the 2-sided 95% confidence interval falls between 1.3 and 1.8, a post-marketing safety trial will be required to document that the true CV risk does not exceed a 30% increase. Finally, medications whose CVOTs result in the upper bound falling below 1.3 may be approved without the need for further documentation of CV safety in post-marketing studies (Figure 3).1 Because documenting only CV safety and not efficacy is required, non-inferiority study design is commonly used. However, outcomes are often additionally assessed for superiority when non-inferiority margins are met.
| Figure 3. United States Food and Drug Administration Evaluation of Diabetes Cardiovascular Outcome Trials1 |
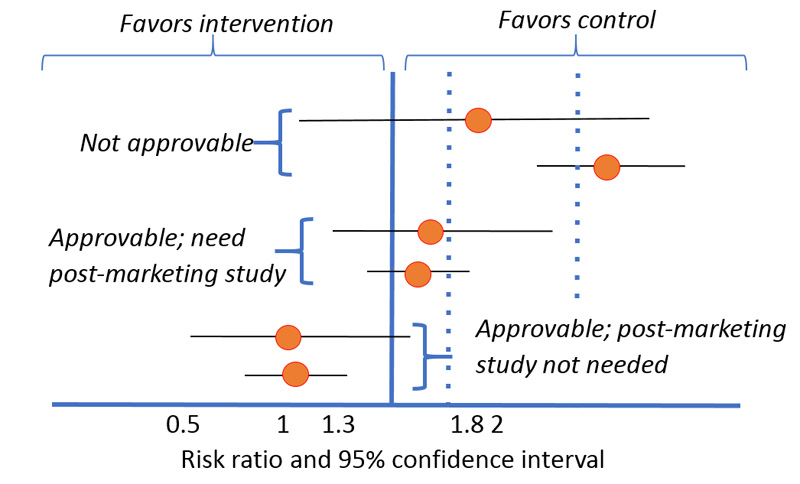 |
| Each CVOT conducted to date has demonstrated at least neutral CV results, while some have also shown benefit for the study medication. Each CVOT varies in terms of study design, population, and duration, which make direct outcome comparisons among the trials difficult. However, these data have shifted the focus of care for those with T2DM from glycemic-centered care to CV-centered care. Guidelines now recommend initial consideration of patients’ CV status when choosing second-line therapies for treating T2DM.25 |
GLP-1 RA CVOTS
Several CVOTs have been conducted for GLP-1 RAs. Overviews of some of the major studies are presented here.
ELIXA: Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome
The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 was the first-reported CVOT for GLP-1 RAs. Its results were published in 2015.
Purpose
ELIXA was conducted to assess the cardiovascular safety of once-daily subcutaneously injected lixisenatide 20 mcg (Adlyxin) by documenting non-inferiority, as well as superiority, for the primary composite outcome of non-fatal myocardial infarction, non-fatal stroke, CV death, or hospitalization for unstable angina (4-point MACE) in an adult population with T2DM and extremely high CV risk.
Enrollment
This multicenter, randomized, double-blind, placebo-controlled trial enrolled 6068 adults with T2DM who had experienced an acute coronary event within 180 days prior to screening. Acute coronary event was defined as a myocardial infarction or hospitalization for unstable angina. Due to inclusion criteria, all participants required secondary prevention of CV disease. Those who met the following criteria were excluded from the trial: estimated glomerular filtration rate (eGFR) of less than 30 mL/min/1.73 m2, age of 30 years or younger, A1C less than 5.5% or greater than 11%, percutaneous coronary intervention within 15 days, coronary artery bypass graft for the qualifying event, or planned coronary revascularization procedure within 90 days after screening.
Outcomes
The study was concluded after a median follow-up of 25 months. At that time, only 5 patients had not received any dose of their assigned treatment. The mean duration of exposure to the study drug was 690 days in the lixisenatide group and 712 days in the placebo group. At that time, 85.5% of patients assigned to the lixisenatide group were taking the maximum dose of 20 mcg, and 96.5% assigned to the placebo group had been dose-escalated to the 20-mcg volume-matched equivalent.
The study population (30.7% women) was predominantly Caucasian (75.4%) with an average age of 60.3 years, a mean duration of diabetes of 9.3 years, an average A1C of 7.7%, and an average eGFR of 76 mL/min/1.73 m2. The majority (83%) had experienced myocardial infarction (non-ST-elevated myocardial infarction, 38.7%; ST-elevated myocardial infarction, 44%) as their qualifying coronary event, while the remainder (17%) had been hospitalized for unstable angina prior to enrollment.
The primary outcome, which was a composite of CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina (4-point MACE), achieved non-inferiority (p<0.001) but failed to show superiority (HR, 1.02; 95% CI, 0.89-1.17; p=0.81), with 13.4% of patients in the lixisenatide group and 13.2% in the placebo group experiencing an event (Figure 4).11 Each individual component occurred at a similar frequency between the 2 groups, further indicating safety yet not demonstrating benefit in any specific CV outcome induced by lixisenatide. The secondary outcome of hospitalizations for HF occurred in 4% and 4.2% of the lixisenatide and placebo groups, respectively (HR, 0.96; 95% CI, 0.75-1.23; p=0.75), indicating a lack of association between lixisenatide and hospitalizations for HF.
| Figure 4. Primary Outcomes and Individual Components of ELIXA11 |
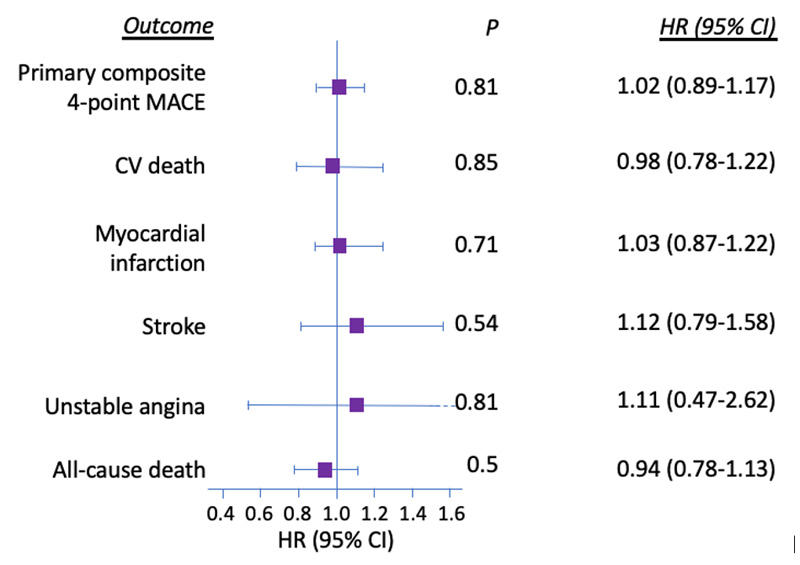 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
ELIXA was the first CVOT to document CV safety for the GLP-1 RA class. This trial was unique in that it evaluated outcomes in an extremely high-CV-risk population, 100% of whom had experienced a recent acute coronary event. This could have, in part, limited achievement of a superior result. The shorter study duration compared to other CVOTs, as well as the pharmacokinetic profile of lixisenatide (its half-life of approximately 3 hours is the shortest of all GLP-1 RAs), which limits its daily durability, could have also influenced the lack of achievement of a superior outcome. Lixisenatide use should be limited to patients with T2DM who would benefit from post-prandial blood glucose control.
LEADER: Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial8 evaluated the long-term effects of liraglutide in patients with T2DM. Its results were published in 2016.
Purpose
LEADER8 was conducted to evaluate CV safety of once-daily subcutaneously injected liraglutide (Victoza) 1.8 mg by documenting non-inferiority and possible superiority in prevention of MACE in a high-risk adult population with T2DM and clinical atherosclerotic cardiovascular disease (ASCVD) (secondary prevention) or pre-established risk factors for CV events. While not the primary focus of the trial, renal outcomes were also assessed.
Enrollment
This multicenter, double-blind, randomized, placebo-controlled study enrolled 9340 adults with T2DM. Patients were generally 50 years of age or older with a baseline A1C of at least 7.0% using standard of care or without previous treatment. Inclusion criteria were divided into 2 categories on the basis of age and CV risk: those 50 years or older with established CV disease (cerebrovascular disease, coronary heart disease, peripheral vascular disease, chronic kidney disease stage 3 or higher, or New York Heart Association [NYHA] class II/III HF) or those 60 years or older with at least 1 pre-determined CV risk factor (microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or decreased blood pressure at the ankles relative to the upper extremities). People living with type 1 diabetes, those with or at risk for multiple endocrine neoplasia type 2 or medullary thyroid cancer, patients who experienced an acute coronary or cerebrovascular event in the previous 14 days, and those using a GLP-1 RA, a DPP-4 inhibitor, pramlintide, or rapid-acting insulin were excluded.
Outcomes
Median follow-up time was 3.8 years. At the conclusion of the trial, 9.5% of patients assigned to liraglutide and 7.3% assigned to placebo had discontinued their assigned drug permanently. Approximately 81% of patients had established CV disease as defined in the 50-years-and-older age group, thus requiring secondary prevention of CV disease. The study population (36% women) had a mean duration of diabetes of 12.8 years and a mean baseline A1C of 8.7%, and 76.8% had an eGFR of at least 60 mL/min/1.73 m2.
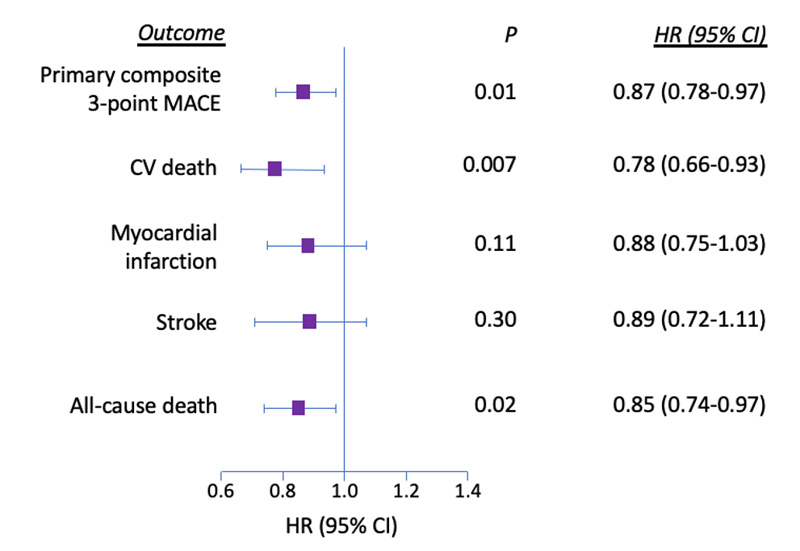
Patients using liraglutide had lower rates of 3-point MACE than patients taking placebo (HR, 0.87; 95% CI, 0.78-0.97). This not only demonstrated safety as being non-inferior (p<0.001) but also demonstrated superiority (p=0.01) at reducing MACE (Figure 5).8 The number needed to treat (NNT) for 3-point MACE was 66, meaning that 66 patients would need to be treated with liraglutide 1.8 mg subcutaneously once daily for 3 years to prevent 1 primary outcome event. Separation of the Kaplan-Meyer curves for the primary composite MACE outcome occurred around 1 year. Each individual outcome favored liraglutide but not all reached statistical significance for superiority on their own. Another noteworthy finding was that the secondary endpoint of all-cause death also occurred less often in patients using liraglutide (HR, 0.85; 95% CI, 0.74-0.97; p=0.02). The Kaplan-Meyer curves began to separate around 18 months of continuous use with liraglutide and resulted in an NNT of 98. Hospitalization for HF was non-inferior (HR, 0.87; 95% CI, 0.73-1.05; p=0.14).
The microvascular secondary endpoint (composite of retinopathy and nephropathy) occurred less often in patients receiving liraglutide (HR, 0.84; 95% CI, 0.73-0.97; p=0.02 for superiority). This was largely driven by the individual component of nephropathy achieving superiority (HR, 0.78; 95% CI, 0.67-0.92; p=0.003). Although not significant, rates of retinopathy occurred more frequently in the liraglutide group than in the placebo group (HR, 1.15; 95% CI, 0.87-1.52; p=0.33).
| Figure 5. Primary Outcomes and Individual Components of LEADER8 |
 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
LEADER was the first CVOT to demonstrate CV benefit (superiority) gained through use of a GLP-1 RA. It specifically documented that daily use of subcutaneous liraglutide in patients with ASCVD or very high CV risk reduced hard outcomes of MACE, CV death, and all-cause mortality compared to standard of care. For these reasons, liraglutide should be considered in patients with T2DM and established ASCVD to further reduce their risks of future MACE. Patients should be educated that these benefits are independent of glycemic improvements and are realized only after 12 to 18 months of continuous use. Secondary outcomes suggest that liraglutide at a 1.8-mg subcutaneous dose once daily may also slow progression of renal decline.
SUSTAIN-6: Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes
The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6)13 evaluated 2 doses of weekly semaglutide versus placebo. Its results were published in 2016.
Purpose
SUSTAIN-613 aimed to evaluate the CV safety of once-weekly subcutaneous semaglutide (Ozempic) compared to placebo as related to the occurrence of MACE in adult patients with T2DM and clinical ASCVD or risk factors for CV events. Of note, SUSTAIN-6 was designed to prove non-inferiority; analysis for superiority was not pre-specified.
Enrollment
This randomized, double-blind, placebo-controlled, parallel-group study enrolled 3297 adults with T2DM to semaglutide 0.5 mg, semaglutide 1.0 mg, or volume-matched placebo. Like many other GLP-1 RA CVOTs, the inclusion criteria of SUSTAIN-6 were determined on the basis of age and CV status. Participants could either be aged 50 years or older with established CV disease, chronic HF (NYHA II or III), or chronic kidney disease stage 3 or higher, or be aged 60 years or older with at least 1 CV risk factor. Those with a history of an acute coronary or cerebrovascular event within 90 days prior to randomization, planned artery revascularization, or on long-term dialysis were excluded from the trial. Other exclusion criteria were treatment with a GLP-1 RA or insulin (other than basal or premixed) within 90 days or treatment with a DPP-4 inhibitor within 30 days prior to screening.
The primary outcome was first occurrence of 3-point MACE, defined as a non-fatal myocardial infarction (including silent), non-fatal stroke, or cardiovascular death. A pre-specified, expanded CV composite included the 3-point MACE plus revascularization, hospitalization for unstable angina, or hospitalization for HF. Additional secondary outcomes included retinopathy complications and new or worsening nephropathy.
Outcomes
Participants had an average age of 64.6 years, a mean duration of diabetes of 13.9 years, an average A1C of 8.7%, and average blood pressure of 135.6/77 mmHg. Of the enrolled patients, 83% had established CVD, 10.7% had chronic kidney disease only, and 17% were 60 years of age or older with only CV risk factors. According to these demographics, most patients enrolled in SUSTAIN-6 required secondary prevention of CV disease, with only 17% requiring primary prevention.
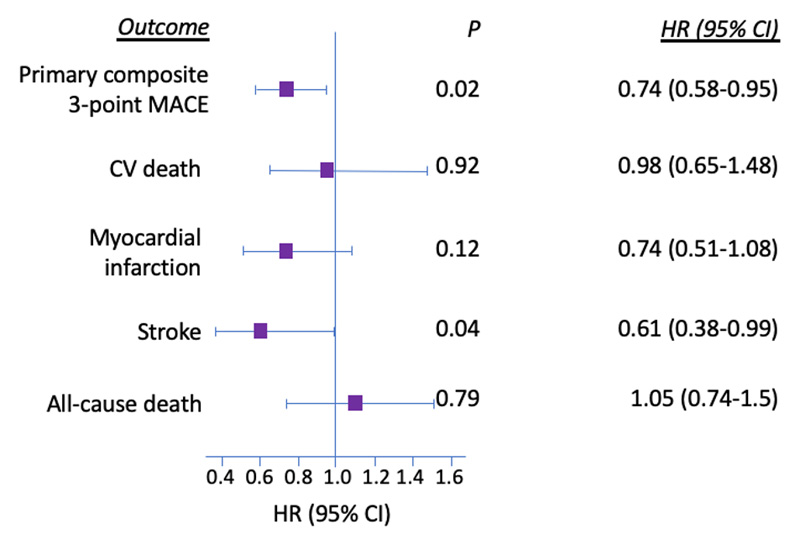
After a median follow-up of 2.1 years, fewer patients randomized to the combined semaglutide group experienced MACE (6.6%) compared to those randomized to the placebo groups (8.9%) (HR, 0.74; 95% CI, 0.58-0.95), which confirmed non-inferiority (p<0.001) (Figure 6).13 Although analysis for superiority was not pre-specified for the primary outcome, it was statistically achieved (p=0.02) with an NNT of 45 for 2 years. All individual components were non-inferior, with a statistically significant reduced benefit of non-fatal stroke (HR, 0.61; 95% CI, 0.38-0.99; p=0.04). CV benefits did not appear to be dose-dependent, as similar risk reductions for 3-point MACE were observed for the semaglutide 0.5-mg (HR, 0.77; 95%, CI 0.55-1.08) and 1.0-mg (HR, 0.71; 95% CI, 0.49-1.02) doses. Semaglutide also lowered the risk of the expanded CV composite (HR, 0.74; 95% CI, 0.62-0.89; p=0.002), which was predominantly due to reductions in revascularization (HR, 0.65; 95% CI, 0.5-0.86; p=0.003), with no significant impact from hospitalization for unstable angina (HR, 0.82; 95% CI, 0.47-1.44; p=0.49) or HF (HR, 1.11; 95% CI, 0.77-1.61; p=0.57).
Evaluation of microvascular outcomes revealed that patients randomized to semaglutide experienced a 36% risk reduction in new or worsening nephropathy (HR, 0.63; 95% CI, 0.46-0.88; p=0.005), defined as persistent macroalbuminuria, doubling of the serum creatinine level and a creatinine clearance less than 45 mL/min/1.73 m2, or the need for continuous renal replacement therapy. Lastly, a statistically significant increased risk of diabetic retinopathy was also noted (HR, 1.76; 95% CI, 1.11-2.78; p=0.02), although the number of events was low (50 in the semaglutide groups and 29 in the placebo groups).
| Figure 6. Primary Outcomes and Individual Components of SUSTAIN-613 |
 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
SUSTAIN-6 demonstrated CV safety of once-weekly semaglutide with clinically meaningful benefits, possibly weighted by reduced risk of non-fatal stroke, in a high-CV-risk adult population with T2DM. As seen in the LEADER study, results also suggest added renal benefits but, unexpectedly, at a higher risk of diabetic retinopathy complications. Semaglutide is a worthwhile option for patients with T2DM and established ASCVD who prefer a less-frequent dosing option or, due to its more profound impact on A1C compared to other GLP-1 RAs, require greater glycemic control. Patients who are unable to tolerate the 1.0-mg dose should be encouraged that CV benefit is still achieved with the 0.5-mg dose but with fewer gastrointestinal adverse events.
EXSCEL: Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetics
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 evaluated long-term safety and efficacy of once-weekly exenatide in patients with a wide range of CV risks. Its results were published in 2017.
Purpose
EXSCEL was conducted to evaluate CV safety of subcutaneously injected once-weekly extended-release (XR) exenatide (Bydureon) 2 mg compared to placebo in addition to standard care. The study was designed to evaluate both non-inferiority and, potentially, superiority in preventing MACE, defined as non-fatal myocardial infarction, non-fatal stroke, or CV death, in a moderately high-risk adult population with T2DM.
Enrollment
This multicenter, double-blind, randomized, placebo-controlled trial was the largest of all GLP-1 RA CVOTs, enrolling 14,752 adults with T2DM (A1C between 6.5% and 10%). Investigators aimed to enroll a population in which at least 70% had a previous CV event. The pragmatic study design permitted patients to receive the standard of care, defined as up to 3 glucose-lowering agents or insulin with up to 2 agents, including use of SGLT-2 inhibitors. Patients with a history of hypoglycemic events (≥ 2 events requiring professional assistance), an eGFR less than 30 mL/min/1.73 m2, previous treatment with a GLP-1 RA, a baseline calcitonin of greater than 40 ng/L, or at risk for endocrine neoplasia or medullary thyroid cancer were excluded.
Outcomes
Investigators met their enrollment goal, with 73.1% of patients having experienced previous CV events (defined as major coronary artery disease [70%], ischemic cerebrovascular disease [22%], or atherosclerotic peripheral artery disease [22%]). The study population (38% female) had an average age of 63 years, an average duration of diabetes of 12 years, a mean baseline A1C of 8.0%, and a mean eGFR of 79 mL/min/1.73 m2.
The median follow-up time was 3.2 years, with a median duration of exposure to the study regimen of 2.4 years in the exenatide group and 2.3 years in the placebo group. At trial conclusion, 43% assigned to exenatide and 45.2% assigned to placebo had permanently discontinued treatment: the majority of discontinuations (30.3% and 32%, respectively) were due to patient decision. This represents the poorest quality of treatment persistence among GLP-1 RA CVOTs to date. Overall, participants received the trial regimen 76% and 75% of the time, respectively.
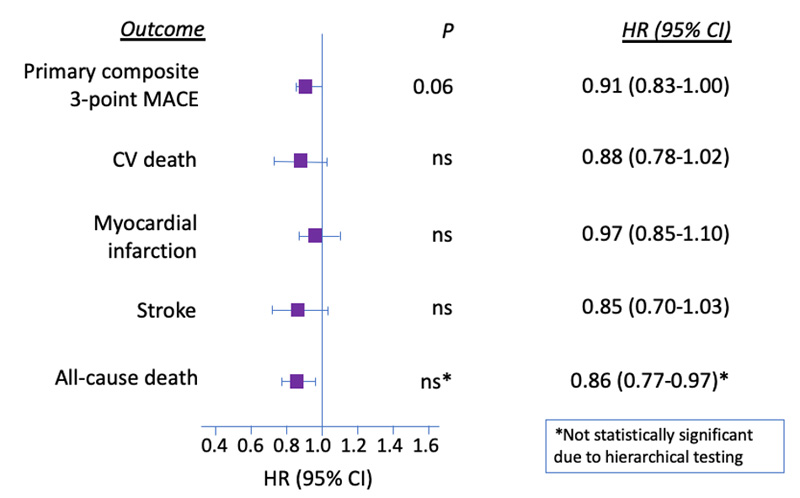
The primary outcome (3-point MACE) achieved non-inferiority (p<0.001) but did not reach superiority (HR, 0.91; 95% CI, 0.83-1.00; p=0.06) (Figure 7).12 Individual components of the 3-point MACE were also non-inferior both per intention-to-treat and per protocol. Uniquely notable was the outcome of death from any cause (HR, 0.86; 95% CI, 0.77-0.97). Although the upper bound of the confidence interval falls below 1.0, it was not deemed to be statistically significant due to hierarchal testing. Renal outcomes were not assessed in this study.
| Figure 7. Primary Outcomes and Individual Components of EXSCEL12 |
 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
EXSCEL documented CV safety of once-weekly exenatide in a moderately high-risk population with T2DM. Several aspects of the study may have significantly influenced the lack of superior outcomes. First, while the study was powered to detect a statistically superior difference in the primary composite outcome, the low treatment persistence limited drug exposure, and, thus, potential impact for detection. Secondly, the pragmatic study design allowed for the evaluation of outcomes under real-world conditions, such as longer follow-up intervals of every 6 months and limited study support, which may further explain the low adherence to treatment. Lastly, EXSCEL utilized the original Bydureon device, which required reconstitution of the dry powder before subcutaneous administration. (Since the conclusion of this study, the manufacturers have developed an improved delivery device for exenatide XR [Bydureon BCise], which does not require reconstitution.) Despite these obstacles, a 9% reduction in the primary outcome was achieved and narrowly missed statistical significance. While superiority was not proven in EXSCEL, results of this trial are reflected in current American Diabetes Association guidelines by listing exenatide XR as the third-best option in the GLP-1 RA class for those with ASCVD behind liraglutide and semaglutide.
PIONEER 6: Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes
The PIONEER program is a set of 10 trials that evaluated oral semaglutide compared to various active medications and/or placebo. PIONEER 610 compared oral semaglutide to placebo. Its results were published in 2019.
Purpose
PIONEER 6 was a phase 3 study performed to establish CV safety of oral semaglutide (Rybelsus) for FDA approval. The goal of the study was to document non-inferiority and possibly superiority to placebo in occurrence of MACE for adults with T2DM and clinical ASCVD or with risk factors for CV events. PIONEER 6 is the most recently published GLP-1 RA CVOT (June 11, 2019) and the first to evaluate an oral GLP-1 RA for occurrence of MACE.
Enrollment
PIONEER 6 was an event-driven, multicenter, international, randomized, double-blind, placebo-controlled study that enrolled 3183 adults with T2DM and high CV risk. Participants were required to be either 50 years of age or older with established CV or chronic kidney disease, or 60 years of age or older with CV risk factors. Patients were excluded from this trial if they met any of the following criteria: treatment with a GLP-1 RA, DPP-4 inhibitor, or pramlintide within the last 90 days; NYHA class IV HF; planned arterial revascularization; myocardial infarction, stroke, or hospitalization for unstable angina or transient ischemic attack within 60 days prior to screening; long-term or intermittent hemodialysis or eGFR less than 30 mL/min/1.73 m2; or proliferative retinopathy or maculopathy. The exclusion of patients with proliferative retinopathy or maculopathy was due to the results observed in SUSTAIN-6, which indicated that weekly subcutaneous semaglutide was associated with a higher risk of diabetic retinopathy than placebo.
In PIONEER 6, patients were randomized to receive either placebo or once-daily oral semaglutide (target dose 14 mg) in addition to standard care. Participants were instructed to take the study drug each morning in a fasting state with up to 120 mL of water and to avoid drinking, eating, or taking other medications for at least 30 minutes thereafter. Semaglutide was initiated at 3 mg once daily, titrated to 7 mg, followed by an increase to 14 mg daily at 4-week intervals to decrease the occurrence of gastrointestinal side effects.26 The primary outcome was first occurrence of MACE from the time of randomization. Like most other CVOTs for GLP-1 RAs, 3-point MACE was a composite of non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death.
Outcomes
Baseline characteristics were similar between the 2 treatment groups. Most patients were male (68.4%) and the population had an average age of 66 years, an average duration of diabetes of 14.9 years, a mean A1C of 8.2%, a mean blood pressure of 136/76 mmHg, and a mean eGFR of 74 mL/min/1.73 m2. The majority (85%) were in the category of at least 50 years of age with established CV or chronic kidney disease. Thus, most enrollees required secondary prevention.
After a median trial duration of 15.9 months, 1347 (84.7%) patients allocated to oral semaglutide and 1435 (90.1%) allocated to placebo completed the trial. By the end of the study, 82.1% randomized to oral semaglutide were using the 14-mg daily dose. More patients randomized to the placebo group intensified therapy with an SGLT-2 inhibitor (7%) than those allocated to oral semaglutide (3.1%).
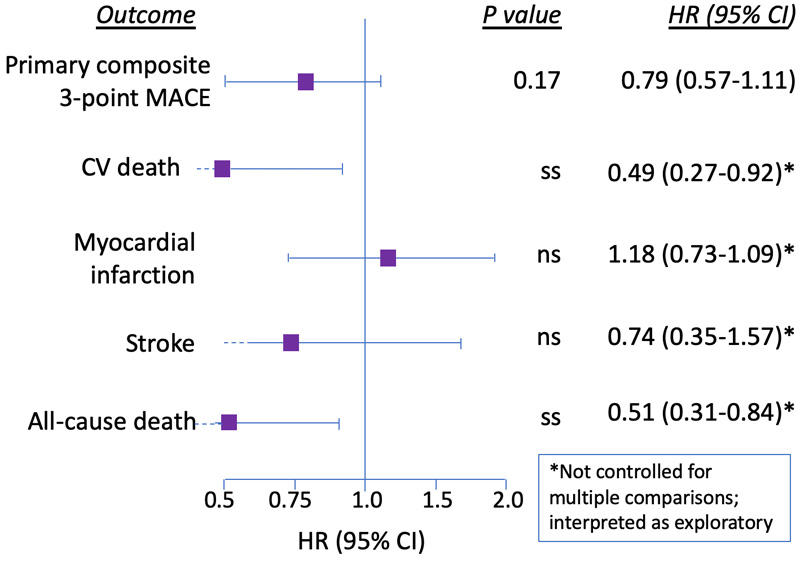
The primary outcome, a composite 3-point MACE, occurred in 3.8% assigned to oral semaglutide and in 4.8% assigned to placebo (HR, 0.79%; 95% CI, 0.57-1.11; p<0.001), thus establishing non-inferiority but not superiority (p=0.17) (Figure 8).10 Although death from CV causes (HR, 0.49; 95% CI, 0.27-0.92) and all-cause mortality (HR, 0.51; 95% CI, 0.31-0.84) appear statistically significant, the authors evaluated the individual components and secondary outcomes only as exploratory, since they did not control for multiple comparisons. The secondary outcome, HF resulting in hospitalization, also met non-inferiority criteria (HR, 0.86; 95% CI, 0.48-1.55). Hospitalization for unstable angina did not meet non-inferiority criteria (HR, 1.56; 95%, CI 0.60-4.01); oral semaglutide use was associated with a 56% increased risk compared to placebo for this endpoint, although it was still only evaluated as exploratory.
| Figure 8. Primary Outcomes and Individual Components of PIONEER 610 |
 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
PIONEER 6 documented CV safety of the first oral GLP-1 RA in a non-inferiority trial. Superiority was not achieved, possibly due, in part, to hindrances of 2-fold greater SGLT-2 inhibitor use in the placebo group than in the treatment group, as similarly observed in the EXSCEL study. Oral semaglutide produced comparable CV outcomes to those observed with once-weekly subcutaneous injections of semaglutide in the similar study population of SUSTAIN-6. Therefore, CV safety of semaglutide appears to be independent of the route of administration. Outcomes from PIONEER 6 allowed for the oral formulation of semaglutide to gain FDA approval in 2019. Patients interested in GLP-1 RA therapy, but who are averse to injections, may prefer using this new formulation of semaglutide with confidence in CV safety.
REWIND: Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes
The Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND) trial9 assessed CV safety of dulaglutide in patients with and without CV disease. Its results were published in 2019.
Purpose
REWIND was conducted to evaluate CV benefit of weekly subcutaneously injected dulaglutide (Trulicity) 1.5 mg. Unlike like other GLP-1 RA CVOTs that initially sought to prove non-inferiority, REWIND was specifically designed to document superiority in preventing MACE compared to standard of care. REWIND also uniquely enrolled a population with the lowest CV risk of any population evaluated by a CVOT.
Enrollment
This multicenter, double-blind, randomized, placebo-controlled trial enrolled 9901 adults with T2DM. Patients were generally 50 years or older with a baseline A1C of 9.5% or lower using standard of care. Enrollees fell into 1 of 3 main categories according to age and CV risk: 50 years or older with established CV disease (defined as a previous myocardial infarction, ischemic stroke, revascularization, hospital admission for unstable angina, or confirmed myocardial ischemia); 55 years or older with myocardial ischemia, coronary, carotid, or lower extremity major artery stenosis exceeding 50%, eGFR less than 60 ml/min/1.73 m2, or albuminuria; and 60 years or older with at least 2 pre-determined CV risk factors (tobacco use, dyslipidemia, hypertension, or abdominal obesity). Those with end-stage renal disease (defined as eGFR < 15 mL/min/1.73 m2), cancer in the past 5 years, life expectancy of less than 1 year, a coronary or cerebral event in the previous 2 months, plans for revascularization, or severe hypoglycemia in the past year were excluded.
Due to planned interim analyses, the p-value required to meet statistical significance was adjusted to 0.0467 to reduce the risk of a type 1 error. Also, the p-value for analyzing individual components of the primary composite endpoint was disproportionately allocated such that 70% of 0.048 (i.e., 0.0336) was used for CV death and 15% of 0.048 (i.e., 0.0072) was used for each of non-fatal stroke and non-fatal MI. Thus, to meet statistical significance, a p-value of 0.034 had to be reached for CV death and a p-value of 0.007 had to be reached for non-fatal stroke and non-fatal myocardial infarction.
Outcomes
After the longest median follow-up of any CVOT to date (5.4 years), 73.2% of patients assigned to dulaglutide and 71.1% assigned to placebo were taking the study drug. Less than one-third of enrollees (31.5%) had established CV disease, and 20% had experienced a previous CV event. Thus, the majority (approximately 69%) required primary prevention of CV disease, representing the largest primary prevention population in a GLP-1 RA CVOT to date. Comparatively, this CVOT also enrolled the largest percentage of women (46%). On average, the study population was 66 years old, had a duration of diabetes of 9.5 years, a baseline A1C of 7.3%, and an eGFR of 75 mL/min/1.73 m2.
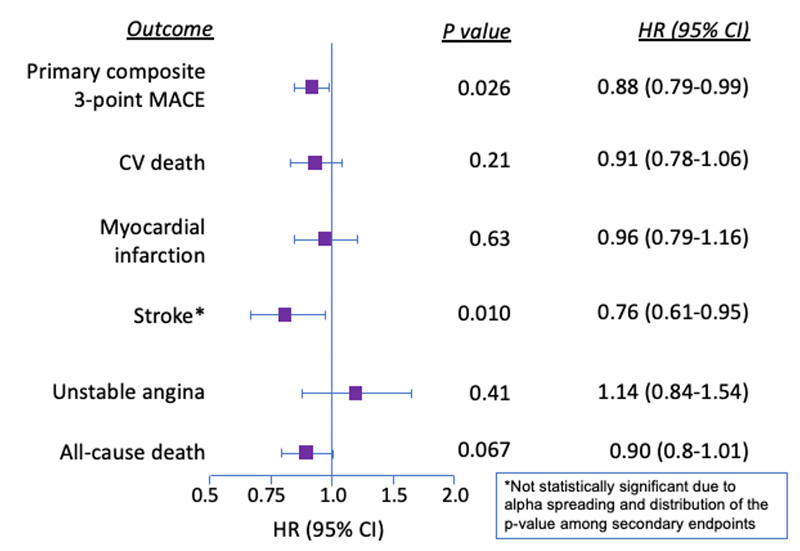
Patients using dulaglutide had lower rates of the primary composite 3-point MACE, which was superior to those using placebo (HR, 0.88; 95% CI, 0.79-0.99; p=0.026) (Figure 9).9 The NNT to prevent 1 of the composite events for patients in the lowest-risk category (primary prevention) was 60. Comparatively, the NNT for patients requiring secondary prevention was impressively small at 18. Similar to other statistically significant findings of GLP-1 RA CVOTs, separation of the Kaplan-Meyer curves for the primary composite MACE outcome occurred around 1 year. Each component of the primary outcome favored dulaglutide. Although none were statistically significant on the basis of the study design, non-fatal stroke (HR, 0.76; 95% CI, 0.61-0.69; p=0.017) gave the greatest weight towards the superior primary composite result. Subgroup analysis demonstrated equivalent CV benefit to populations regardless of baseline A1C (< 7.2% vs. > 7.2%), age, sex, body mass index, duration of diabetes, or geographical region.
Notably, the secondary composite renal outcome (new macroalbuminuria, a sustained decline in eGFR of ≥ 30% from baseline, or chronic renal replacement therapy) occurred less often in patients assigned to dulaglutide than in those assigned to placebo, which was statistically significant (HR, 0.85; 95% CI, 0.77-0.93; p=0.0004) with an NNT of 31. This benefit was driven by a reduced onset of new macroalbuminuria (HR, 0.77; 95% CI, 0.68-0.87; p<0.0001).27 However, the composite eye outcome (photocoagulation, anti-vascular endothelial growth factor therapy, or vitrectomy) was not statistically significant (HR, 1.24; 95% CI, 0.92-1.68; p=0.16).
| Figure 9. Primary Outcomes and Individual Components of REWIND9 |
 |
| CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular event. |
Conclusions
REWIND was the first and only CVOT to date to document CV benefit in a particularly low-risk population needing only primary prevention. Patients requiring secondary prevention (approximately 30% of the study population) also benefited from once-weekly subcutaneously administered dulaglutide. Because of this CVOT, dulaglutide should be the selective GLP-1 RA for patients without CV disease. Further, the dose of dulaglutide should be increased to the studied 1.5-mg dose after adequate use of the starting dose. Secondary and exploratory outcomes suggest that the use of dulaglutide may help preserve renal function, particularly by preventing development of new macroalbuminuria.
GLP-1 RA CVOT DISCUSSION
New mandates set forth by the FDA for manufacturers to evaluate CV safety when submitting an NDA have produced significant clinical implications as a direct result of drastically different investigations than those previously performed when pursuing FDA approval in T2DM. Now, meaningful outcomes beyond A1C and glucose control in the form of MACE are the primary endpoints, and enrolled studied populations better represent those treated in practice. These recent CVOTs included patients with long-standing diabetes (9-15 years), advanced age (60-66 years), some degree of renal impairment (21%-28% with eGFR < 60 ml/min/1.73 m2), and with varying degrees of ASCVD (Table 1).8-13 Further, these CVOTs followed patients for longer periods of time, ranging from 1.3 years in PIONEER 610 to 5.5 years in REWIND9, and provide a substantial number of patient-years of GLP-1 RA exposure to analyze for CV safety. Thus, these CVOTs give confidence for long durations of therapy with GLP-1 RAs in patients who are truly representative of those commonly treated with T2DM.
| Table 1. Comparison of GLP-1 RA CVOT Study Populations8-13 |
TRIAL
Drug |
ELIXA11
Lixisenatide |
LEADER8
Liraglutide |
SUSTAIN-613
Semaglutide SQ |
PIONEER 610
Semaglutide PO |
EXSCEL12
Exenatide XR |
REWIND9
Dulaglutide |
| Median follow-up time, y |
2.1 |
3.8 |
2.1 |
1.3 |
3.2 |
5.5 |
| Trial participants, n |
6068 |
9340 |
3297 |
3183 |
14752 |
9901 |
| Mean age, y |
60.3 |
64.3 |
64.6 |
66 |
62.0 |
66 |
| Female sex, n (%) |
2894 (30.7) |
3337 (35.7) |
1295 (39.3) |
1007 (31.6) |
5603 (38.0) |
4589 (46.3) |
| Established ASCVD, n (%) |
6068 (100) |
6775 (72.5) |
2735 (83.0) |
2695 (84.7) |
10,782 (73.1) |
3114 (31.5) |
| History of HF, n (%) |
1922 (20.3) |
1667 (17.8) |
777 (23.6) |
388 (12.2) |
2389 (16.2) |
853 (8.6) |
| eGFR < 60 mL/min/1.73 m2, n (%) |
1407 (23.2) |
2158 (23.1) |
939 (28.5) |
875 (27.5) |
3191 (21.6) |
2199 (22.6) |
| Mean duration of diabetes, y |
9.3 |
12.9 |
13.9 |
14.9 |
12.0 |
10.0 |
| Mean baseline A1C, % |
7.7 |
8.7 |
8.7 |
8.2 |
8.0 |
7.3 |
| A1C, glycated hemoglobin; ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; ER, extended release; HF, heart failure; PO, by mouth; SQ, subcutaneously. |
In addition to establishing CV safety in a more real-world population with T2DM, CVOTs have also produced noteworthy and clinically impactful findings of several GLP-1 RAs that would not have been revealed otherwise. Specifically, LEADER,8 SUSTAIN-6,13 and REWIND9 documented statistically significant reductions in the rates of MACE by their studied GLP-1 RA compared to placebo. Outcomes from LEADER have allowed liraglutide to gain an FDA-labeled indication “to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.”28 Though at the time of this publication, no other GLP-1 RA has received a CV-specific FDA indication, similar approvals may be forthcoming, given the results of recent CVOTs. In response to these observations of CV benefit rather than just safety, guidelines for the treatment of patients with T2DM have changed dramatically.25
The focus of T2DM treatment has shifted from glycemic improvement and adverse event mitigation towards initial evaluation of patients for ASCVD, which should accordingly influence second-line therapy selection. Guidelines currently recommend GLP-1 RA selection on the basis of CVOT outcomes with strongest CV evidence in the order of liraglutide > subcutaneous semaglutide > exenatide XR. However, publication of the REWIND9 and PIONEER 610 studies may influence this tiered order. Further, REWIND uniquely offers evidence of benefit to patients without ASCVD in need of primary CV prevention. Thus, future guideline recommendations may shift to include considerations of dulaglutide in expanded populations with lower CV risks. Still, GLP-1 RAs with evidence of CV benefit should be selected over therapies with proof of CV safety alone or even without established CVOT outcomes at all.
Since CV outcomes occur irrespective of glycemic control, patients and providers should be encouraged to continue well-tolerated, guideline-recommended GLP-1 RA therapy even when glycemic benefits are not as evident. Similarly, individuals who are intolerant of higher doses of GLP-1 RAs will likely still gain CV benefit if a lower dose is used to mitigate adverse gastrointestinal events, as evidenced by SUSTAIN-6,13 which demonstrated a lack of dose-dependent CV benefit with semaglutide. While the mechanism of CV benefit for this medication class is unclear, it appears to occur through myriad processes, all of which are independent of glucose-lowering abilities. Mechanisms of eliciting CV benefits may include decreased production of cardiac and vascular inflammatory markers, modified progression of atherosclerotic vascular disease, and improved endothelial function through vasodilation.29 These proposed mechanisms help explain the 12 to 18 months of continuous use required to elicit the CV benefit from GLP-1 RAs with statically significant outcomes.
Despite these new insights into CV benefits of GLP-1 RAs, questions still remain. Presently, it is not possible to declare a class effect on CV impact due to the inconsistent outcomes among CVOTs. However, studies with only neutral (non-inferior) results each have limitations that could have influenced the outcomes. Studies with a pragmatic design, high discontinuation rates, or shorter study durations could have limited overall exposure to the study GLP-1 RA, which ultimately could have dampened the ability to detect a difference if one existed. Also, since SGLT-2 inhibitors are also known to have significant CV benefits, studies with more SGLT-2 inhibitor use in the comparator arm could have prevented the ability to detect a difference produced by the study GLP-1 RA.
CVOTs of other classes have also provided insight for clinical use. All CVOTs for DPP-4 inhibitors have documented safety related to MACE, but without gained benefit.4-7 Saxagliptin was found to increase the frequency of hospitalizations for HF (HR, 1.27; 95% CI, 1.07-1.51; p=0.007)4 and alogliptin appeared to trend in that same direction (HR, 1.07; 95% CI, 0.79-1.46).5 Conversely, all CVOTs for SGLT-2 inhibitors have demonstrated CV benefit in reducing MACE and frequency of hospitalization for HF.14-16 Dapagliflozin was uniquely studied in a lower-CV-risk population.16 The primary 3-point MACE endpoint did not achieve statistical significance (HR, 0.93; 95% CI, 0.84-1.03; p=0.17), but this was likely influenced by enrollment of the lower-risk population. In a subsequent investigation, dapagliflozin specifically showed benefit in reducing the risk of worsening HF and CV death in patients with established systolic HF with or without T2DM (HR, 0.74; 95% CI, 0.65-0.85; p<0.001).30 In each of these SGLT-2 inhibitor studies, CV benefit appears with a faster onset than with GLP-1 RAs, appearing at around 1 to 3 months. Mechanisms for SGLT-2 inhibitor CV benefits are different from those proposed to influence GLP-1 RA benefits: intravascular volume contraction, decreased cardiac afterload and preload, and improved systolic and diastolic function.29 Also, following the renal-focused study CREDENCE, canagliflozin has documented ability to slow progression of renal decline in patients with low baseline eGRF and persistent proteinuria.31 The relative risk of the composite renal outcome was 34% lower in the canagliflozin group than in the placebo group (HR, 0.66; 95% CI, 0.53-0.81; p=0.001). While the REWIND,9 LEADER,8 and SUSTAIN-613 studies also showed reductions in composite renal outcomes, these secondary endpoints were noted to be influenced predominantly by a decline in macroalbuminuria when further analyzed.27
Lastly, no randomized trials yet exist specifically investigating the utility of combining a GLP-1 RA and an SGLT-2 inhibitor in T2DM. Because the proposed mechanisms are distinctly different for both glycemic improvements and CV risk reductions, the benefit of combining the use of these 2 classes can be speculated. This combination would theoretically provide a relatively fast onset of CV benefit (1-3 months) by the SGLT-2 inhibitor with potentially further augmented reductions in MACE exhibited 12 to 18 months later as a result of the GLP-1 RA.
Ultimately, this new information regarding CV outcomes in the treatment of T2DM can and should be used to drive therapeutic selection for treatment. In addition to pharmacokinetic profile, glycemic impact, delivery device, side-effect profile, route of administration, and frequency of dosing, prescribers should also consider the CV outcomes of CVOTs in the context of the studied population.
REFERENCES
- United States Food and Drug Administration. Guidance for Industry: Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes Mellitus. https://www.fda.gov/media/71297/download. Published December 2008. Accessed November 21, 2019.
- ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701-8.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457-71.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-26.
- White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327-35.
- Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-42.
- Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-22.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121-30.
- Husain M, Birkenfeld AL, Donsmark M, et al; PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-51.
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247-57.
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-39.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-44.
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-57.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-28.
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-57.
- Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789-830.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-53.
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-89.
- Nathan DM, Cleary PA, Backlund JYC, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-53.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-72.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-39.
- Home PD, Pocock SJ, Beck-Nielsen H, et al; RECORD Study Team. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125-35.
- Benjamin EJ, Muntner P, Chair Alonso A, et al; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Circulation. Circulation. 2019;139(10):e56-528.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes--2019. Diabetes Care. 2019;42(Supplement 1):S90-S102.
- Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: Rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21(3):499-508.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131-8.
- Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk;2019.
- Sattar N, Petrie MC, Zinman B, Januzzi JL. Novel diabetes drugs and the cardiovascular specialist. J Am Coll Cardiol. 2017;69(21):2646-56.
- McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
- Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-306.
Back Top