
Expired activity
Please go to the PowerPak
homepage and select a course.
Granulocyte-Colony Stimulating Factor Biosimilars: An Update for Frontline Oncology Practitioners (Audio-enriched Monograph)
» Patient Handout (PDF)
INTRODUCTION
Cancer care costs have increased rapidly over the last decade, from $88.3 billion in 2011 to an estimated $173 billion in 2020.1 Biologics, including cancer therapies, represent a significant proportion of such costs. Total biologic sales in the United States (U.S.) in 2018 were an estimated $125 billion, reflecting a 50% increase over 2014.2 Global sales of 3 leading oncology biologics—trastuzumab, rituximab, and bevacizumab—amounted to $19.4 billion in 2018.3 Greater adoption of new, costly targeted agents and immunotherapies will only further increase cancer care costs, highlighting the need to identify opportunities to reduce drug expenses where possible.
One approach that could decrease cancer biologic cost is through expanded biosimilar use. A biosimilar is a biologic medicine proven to be highly similar to an approved reference product (originator), with comparable efficacy and safety. The number of marketed biosimilars is rapidly growing in the U.S. and globally. The European Medicines Agency (EMA) approved the first biosimilar in 2006 for somatropin. By the end of 2019, over 260 biosimilars were approved globally, with oncology biosimilars comprising more than half of these products.4
Biosimilar adoption has been greater in the European Union (EU) compared to the U.S., in part due to differences in regulatory and patent processes. Biologics in the EU are often procured through government contracts to reduce expenditures. While biosimilar use in the U.S. is rising, currently they comprise an estimated 2.3% of all biologics in the country compared to 90% in the EU. This is surprising given that 60% of all biologic sales occur within the U.S.5 As of December 2020, the U.S. Food and Drug Administration (FDA) had approved 28 biosimilars (including 15 oncology products) , and more are expected.6 Key approved oncology biosimilars include those for trastuzumab, rituximab, and bevacizumab. Additionally, biosimilars for several hematopoietic growth factors have been approved, including those for granulocyte-colony stimulating factor (G-CSF) and epoetin. Biosimilars for noncancer therapies, such as those targeting tumor necrosis factor (e.g., etanercept, adalimumab), are approved but not yet marketed in the U.S.
Greater clinical adoption of oncologic and supportive care biosimilars could result in substantial cost savings to patients, particularly as many biologics come off patent and biosimilars replace them.7 Depending on the degree to which biosimilars are incorporated into practice, they may also lower the overall cancer care cost burden on the U.S. healthcare system. Additionally, cost savings realized with biosimilar use could allow for expanded patient access to expensive life-saving cancer therapies (e.g., targeted agents and immunotherapy) that otherwise might be unaffordable. The EU, which has wider use of biosimilars, has experienced both lower cost and improved patient access.8 Finally, biosimilar’s reduced drug development and production costs, coupled with the shorter time required for approval, could aid pharmaceutical manufacturers given the costly and onerous requirements for new drug development. Oncology biosimilars’ ultimate impact on patient care will depend on their acceptance by providers, payers, and patients. It will require adequate understanding of the similarities and differences of biosimilars from reference products and generics and an appreciation of their safety and efficacy profiles relative to their originators.
G-CSF Indications
Neutropenia is a clinically significant and potentially life-threatening problem for many patients with cancer. Chemotherapy-induced neutropenia (CIN) commonly occurs in patients who receive myelosuppressive cytotoxic chemotherapy.9 Neutropenia and febrile neutropenia (FN) can result in infections, hospitalization, treatment delays, and increased healthcare costs. These toxicities can limit systemic chemotherapy use and substantially impact patient care. Patients undergoing hematopoietic stem cell transplantation or autologous peripheral blood progenitor cell collection and therapy are also at increased risk. In addition to cancer, neutropenia can also co-occur with other diseases or therapies including infections, selected bone marrow disorders, certain drugs, and radiation therapy. The discovery and clinical development of recombinant G-CSF has helped mitigate these effects.
G-CSF is an endogenous lineage-specific hematopoietic cytokine that stimulates neutrophil progenitor production in the bone marrow and enhances neutrophil number and function.10 G-CSF binding to its receptor, which is expressed on various hematopoietic cells, stimulates hematopoietic stem cell mobilization and promotes neutrophil proliferation, differentiation, and function (e.g., phagocytic ability, antibody-dependent killing). Cloning the G-CSF gene led to development of a recombinant human form that was subsequently marketed for clinical use. FDA approved the first exogenous G-CSF, filgrastim, in 1991, followed by pegfilgrastim (pegylated filgrastim) in 2002.11
Recombinant human G-CSF has been used to
- safely and effectively reduce the duration of neutropenia and incidence of FN in patients with nonmyeloid malignancies receiving myelosuppressive chemotherapy
- reduce neutropenia in patients undergoing myeloblastic therapy followed by bone marrow transplantation who are at increased risk of prolonged severe neutropenia
- mobilize peripheral blood progenitor cells
A list of approved G-CSF indications is in Table 1. Filgrastim and pegfilgrastim, therefore, can aid in FN management without the need for chemotherapy dose reductions, thus allowing delivery of full dose-intensity therapy.12 These growth factors also provide substantial economic and social value due to reductions in FN-related hospitalizations and deaths, less antibiotic use, and improvements in health-related quality of life.13
| Table 1. FDA-Approved Indications for G-CSF Originator and Biosimilars89-97 |
| |
Filgrastim |
Pegfilgrastim |
| Indications |
Filgrastim (Neupogen®) 35 |
Filgrastim-sndz (Zarxio®) |
Filgrastim-aafi (Nivestym®) |
Tbo-filgrastim (Granix®) |
Pegfilgrastim (Neulasta®) 34 |
Pegfilgrastim-apgf (Nyvepria™) 50 |
Pegfilgrastim-bmez (Ziextenzo®) |
Pegfilgrastim-cbqv (Udenyca®) |
Pegfilgrastim-jmdb (Fulphila™) |
| Decrease the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe neutropenia with fever |
✓ |
✓ |
✓ |
✓b |
✓a |
✓a |
✓a |
✓a |
✓a |
| Reduce the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with AML |
✓ |
✓ |
✓ |
✘ |
|
✘ |
✘ |
✘ |
✘ |
| Reduce the duration of neutropenia and neutropenia-related clinical sequelae (e.g.‚ febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by BMT |
✓ |
✓ |
✓ |
✘ |
|
✘ |
✘ |
✘ |
✘ |
| Mobilize autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis |
✓ |
✓ |
✓ |
✘ |
|
✘ |
✘ |
✘ |
✘ |
| Reduce the incidence and duration of sequelae of severe neutropenia (e.g.‚ fever‚ infections‚ oropharyngeal ulcers) in symptomatic patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia |
✓ |
✓ |
✓ |
✘ |
|
✘ |
✘ |
✘ |
✘ |
| Increase survival in patients acutely exposed to myelosuppressive doses of radiationc |
✓ |
✘ |
✘ |
✘ |
✓ |
✘ |
✘ |
✘ |
✘ |
Abbreviations: AML, acute myeloid leukemia; BMT, bone marrow transplantation; FDA, U.S. Food and Drug Administration; G-CSF, granulocyte-colony stimulating factor.
aDecrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia.
bReduction in the duration of severe neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia.
cHematopoietic Syndrome of Acute Radiation Syndrome. |
The National Comprehensive Cancer Network (NCCN) now considers G-CSF prophylaxis standard therapy for reducing the incidence, duration, and severity of FN induced by myelosuppressive chemotherapy. G-CSF can reduce the time to neutrophil recovery, hospitalization length, and infection-related mortality associated with chemotherapy. Current treatment guidelines support G-CSF prophylaxis use if FN risk is greater than 20% since G-CSF therapy cost is thought to outweigh potential costs of hospitalization and FN treatment.12,14,15 Due to the coronavirus disease (COVID)-19 pandemic, the NCCN expanded these guidelines to also include patients at intermediate FN risk (i.e., 10% to 20% risk).16 Providers usually start filgrastim or pegfilgrastim administration within 24 to 72 hours following completion of myelosuppressive chemotherapy. They administer filgrastim daily for several days to reduce cytotoxic effects on progenitor cells stimulated by G-CSF. Studies suggest, however, that higher drug pricing may limit filgrastim and pegfilgrastim access for some patients. Additional treatment options that could facilitate G-CSF therapy, possibly at a lower cost, would be desirable.
BIOSIMILARS OVERVIEW
The Biologics Price Competition and Innovation Act (BPCIA) first established biosimilars in the U.S. in 2009. Under the BPCIA, biosimilarity is defined to mean that “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components” and “there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product” (Table 2).17 It must be emphasized that biosimilars are not the same as generic drugs. Generic drugs are medications that contain a chemically synthesized active pharmaceutical ingredient that is identical to that found in the original drug. Generics are defined as “a medication created to be the same as an existing approved brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics.”18 Biosimilars are not chemically created but are biological products derived from living cellular systems.
| Table 2. Definitions15 |
| Term |
Definition |
Reference Product
(comparison) |
Substitution Statute* |
MD-Initiated
Changea |
Pharmacist-
Initiated
Changeb |
| Generic |
Must be pharmaceutically equivalent and bioequivalent |
Innovator brand: All products deemed equivalent to a brand may also be deemed equivalent to other therapeutic equivalents |
State-regulated
authorization of
generic
substitution |
Yes |
Yes, in most states |
| Reference product |
Single licensed biologic product against which a biologic product is evaluated in a 351(k) application |
|
|
|
|
| Biosimilar |
Highly similar to an already FDA-approved biologic product, and shown to have no clinically meaningful differences from reference product |
Reference biologic: Biosimilars are deemed biosimilar to the reference product only |
|
Yes |
No |
| Interchangeable |
Meets definition of biosimilars andbiosimilarity standard, and is expected to produce the same clinical result as reference product in any given patient for a biologic product that is administered more than once to an individual; risk in terms of safety, or diminished efficacy of alternating or switching between use of the biologic product and the reference product, is not greater than the risk of using the reference product without such alternation or switching |
Reference biologic: Interchangeability of a product indicates interchangeability with reference biologic only |
BPCIA; FDA-deemed interchangeable products may be dispensed in place of reference product |
Yes |
Yes |
Abbreviations: BPCIA, Biologics Price Competition and Innovation Act; FDA, U.S. Food and Drug Administration.
aThe physician may always choose which products to prescribe, administer, or dispense to the patient. Product selection is not regulated by any federal or state body, but rather reflects the physician’s judgement regarding which product will result in desired outcomes—that is, physicians may use data, FDA determinations, etc, to understand equivalence and expected clinical outcomes.
bThe most restrictive states prohibit any substitution without express consent of the physician. The least restrictive states mandate substitution if there is an FDA-approved therapeutic equivalent. Most states require patient notification in any situation in which a product is substituted.
[Reproduced from: Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: Biosimilars in oncology. J Clin Oncol. 2018;36(12):1260-1265.]15 |
In contrast with generic drugs, which have a low molecular weight and reproducible structure, biosimilars are typically complex, high-molecular-weight molecules. Because of their inherent biological variability, they can vary with respect to post-translational modifications, protein folding, and other parameters. Therefore, slight differences can exist between a biosimilar and its reference compound (originator) and between multiple versions of biosimilars.19 As discussed below, biosimilars have a unique nomenclature and regulatory review process that further differentiates them from generic drugs.
Nomenclature
FDA has proposed a unique naming system for biosimilars. Biosimilars have a nonproprietary core name of the reference biologic (e.g., filgrastim) plus an FDA-designated distinguishing suffix of 4 random lower-case letters (e.g., -sndz, -aafi) that is devoid of meaning.20 Suffixes may not include abbreviations commonly used in clinical practice, which could lead to prescription errors. They also cannot be too similar to any other FDA-designated nonproprietary name suffix and should not appear similar to or connote the name of the license holder. This system is designed to help healthcare professionals differentiate these products from their originators and related biosimilars and to facilitate pharmacovigilance. Such nomenclature may also aid providers in distinguishing among multiple biosimilars with similar-sounding names that could slightly differ from one another in terms of safety.
FDA Review and Approval Process for Biosimilars
Like originator biologics, FDA subjects biosimilars to a thorough regulatory review process prior to approval. Biosimilars must undergo rigorous preclinical and clinical evaluations, after which FDA reviews the totality of evidence prior to approval (Figure 1).21 In contrast with originator compound approval, which places more weight on clinical trial results, biosimilar regulatory review involves greater emphasis on immunogenicity, pharmacokinetic/pharmacodynamic (PK/PD) data, and physicochemical properties. Analytic assays are required to fully characterize a biosimilar’s identity, concentration, purity, potency, and consistency. FDA permits slight variations from the originator compound with respect to physicochemical properties (e.g., glycosylation, higher-order protein structure, primary sequence) that may arise from differences in post-translational modifications, manufacturing process, or purification. A biosimilar must, however, be identical to the originator in terms of its dosage form, mechanism of action, PK/PD, route of administration, and strength. There must also be “no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency.”22
Figure 1. Typical Regulatory Assessment Process for Biosimilars21
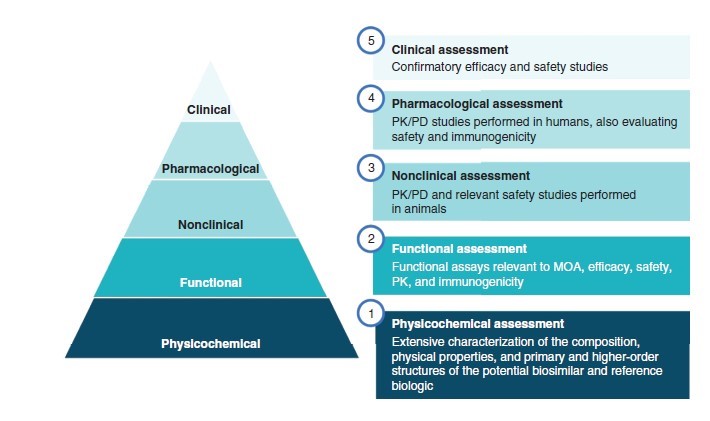
Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics; MOA, mechanism of action.
[Reproduced from: Waller CF, Friganović A. Biosimilars in oncology: key role of nurses in patient education.
Future Oncol. 2020;16(25):1931-1939.]21
Extrapolation
In certain cases, FDA may grant extrapolation to support biosimilar licensure to 1 or more closely related indications of its originator product without the need for additional clinical testing. The manufacturer must submit data for each new indication requested to support the claim that the biosimilar will be as active as the reference product. Extrapolation of indications is based on efficacy and safety data for the biosimilar and consideration of its endpoints, mechanism of action, patient- and disease-specific factors, and other evidence. This allows for expansion of biosimilar use without the need to conduct additional clinical trials in those indications. For example, FDA originally evaluated and approved filgrastim-sndz for prevention of severe neutropenia associated with chemotherapy for breast cancer. They later extrapolated this approval to include other approved indications for the filgrastim originator (e.g., neutropenia related to bone marrow transplantation, severe chronic neutropenia).23,24
“Skinny labeling” (carve-outs) allows drug manufacturers to seek approval for some (but not all) of the approved indications for a reference drug. Manufacturers have used this as a means to avoid patent litigation around specific indications by copying only the parts of the brand label corresponding to nonpatented indications.25 The potential application of skinny labeling for biosimilars is still under discussion. One concern is that if a biosimilar is not approved for all of the same indications as its originator, some providers might believe that it is not a real biosimilar. They could believe it has a different mechanism of action or other drawbacks relative to its originator, leading to lower acceptance and use.15
Substitution and Interchangeability
Most states have enacted their own legislation regulating pharmacist substitution of biosimilars for reference products.26 Specific regulations vary by state but often involve27
- provider notification regarding substitution
- option for nonsubstitution
- legal immunity for pharmacists
- patient notification and an explanation of any cost or pricing differences they may realize
- maintenance of a list of FDA-approved interchangeable products
- record maintenance for a defined time period
Most state laws, though, limit final treatment decisions to the prescriber, including the prescribing of biosimilars or prevention of substitution.
Some healthcare professionals may be concerned about switching from an originator to a biosimilar since they want to ensure that the drugs are equivalent in efficacy, immunogenicity, and safety. In theory, product drift—unintended or unexplained deviations in manufacturing processes that can lead to changes in product attributes—might occur that could alter efficacy and/or safety. To date, however, these concerns have not been borne out with G-CSF biosimilars. Researchers conducted a study comparing use of filgrastim originator to a biosimilar (filgrastim-sndz) for severe neutropenia prevention in patients receiving myelosuppressive chemotherapy for breast cancer. They found no clinically meaningful differences in efficacy, safety, or immunogenicity when switching from reference product to biosimilar or vice versa.28 These results are supported by a systematic review of 90 studies (including 7 filgrastim trials) that involved switching from reference drugs to biosimilars, encompassing 14 disease indications and 14,225 patients. Most studies found no substantial differences in safety (including immunogenicity) or efficacy when switching between reference products and biosimilars. This provides support for the ability to switch between a G-CSF originator and its biosimilar and minimizes immunogenicity concerns.29
Separate regulatory requirements established under the BPCIA 351(k)(4) pathway exist for interchangeable biosimilars. Biosimilars designated as interchangeable have to meet additional, more stringent requirements. By definition, an interchangeable biosimilar must be “expected to produce the same clinical results as the reference product in any given patient.”30 Switching back and forth between an interchangeable biosimilar and originator should not alter safety, efficacy, or PK/PD. Laws regulating interchangeable biosimilars vary by state, but reference product substitution with an interchangeable biosimilar generally requires prescriber approval (similar to prescribing small-molecule generic drugs). To date, however, FDA has not approved any interchangeable biosimilars. Draft FDA guidelines propose that industry applicants can also request that their drugs be classified as a biosimilar but without interchangeability.30
G-CSF BIOSIMILARS
Regulatory agencies have approved biosimilars for the 2 types of G-CSF, filgrastim and pegfilgrastim. The 2008, the EMA approved the first G-CSF biosimilar for filgrastim. FDA approved its first G-CSF biosimilar, filgrastim-sndz, in 2015 following demonstration of its equivalence to the originator compound with respect to efficacy, immunogenicity, PK/PD, protein structure, receptor binding, and safety. This biosimilar’s approval covered all of the same indications as its originator product.10
Currently, 6 G-CSF biosimilars are FDA-approved, including 2 short-acting compounds and 4 pegylated long-acting ones (Table 3). A systematic review and meta-analysis found no clinically significant differences between short- and long-acting G-CSFs for reduction of chemotherapy-induced FN when dosed according to recommended guidelines.31 FDA approved another short-acting recombinant G-CSF, tbo-filgrastim, as an original biologic prior to establishment of their biosimilar process. While similar to the reference product with respect to efficacy, PK, and safety, it is not considered a biosimilar per se, so its indication is more limited.32 In addition to these FDA-approved products, the EMA has approved another 8 filgrastim and 5 pegfilgrastim biosimilars.33
| Table 3. G-CSF Biosimilars Currently Approved in the United States |
| Product |
Description |
Approval Date |
Filgrastim
Tbo-filgrastima (Granix®)92
Filgrastim-sndz (Zarxio®)90
Filgrastim-aafi (Nivestym®)91 |
Short-acting
Short-acting
Short-acting
|
2012
2015
2018 |
Pegfilgrastim
Pegfilgrastim-cbqv (Udenyca®)96
Pegfilgrastim-jmdb (Fulphila™)97
Pegfilgrastim-bmez (Ziextenzo®)95
Pegfilgrastim-apgf (Nyvepria™)50 |
Long-acting
Long-acting
Long-acting
Long-acting |
2018
2018
2019
2020 |
| aTbo-filgrastim initially approved as an original biologic prior to establishment of FDA biosimilar process; approved in 2012 for severe neutropenia. |
PEGylation (conjugating polyethylene glycol [PEG] to a target) can stabilize a protein’s half-life in circulation and reduce its clearance. Pegylated filgrastim’s half-life (pegfilgrastim, a PEGylated G-CSF) can thus be extended to 15 to 80 hours (compared with 3 to 4 hours with filgrastim).34,35 In 2002, FDA approved pegfilgrastim for reducing the infection and FN incidence in patients with nonmyeloid malignancies receiving myelosuppressive systemic anticancer therapy.24 Pegfilgrastim administration may be more convenient for some patients since fewer hospital visits and tests to measure absolute neutrophil count (ANC) are needed compared to filgrastim therapy. Additionally, providers can administer pegfilgrastim as single dose rather than daily, reducing infusion requirements and enhancing compliance.
The American Society of Clinical Oncology (ASCO) guidelines on hematopoietic growth factor use now include filgrastim and pegfilgrastim biosimilars and their reference compounds for FN prophylaxis in patients with cancer.36 The NCCN guidelines also state that “an FDA-approved biosimilar is an appropriate substitute for filgrastim and pegfilgrastim” for prophylactic neutropenia management in patients receiving myelosuppressive chemotherapy and for hematopoietic stem cell mobilization.14 Some G-CSF biosimilars (filgrastim-sndz and filgrastim-aafi) are approved for the same indications as the reference product (except for Hematopoietic Syndrome of Acute Radiation Syndrome), whereas others have more limited approved uses (Table 1).
Providers should note that while both G-CSF and granulocyte-macrophage colony stimulating factor (GM-CSF) are molecularly cloned hematopoietic growth factors, substantial differences exist between these products and between their biosimilars. G-CSF and GM-CSF have distinct sequences, glycosylation, structures, receptors, mechanisms of action, efficacy, and indications.37 Consequently, these growth factors should not be considered clinically interchangeable.
Clinical Trials
Researchers have conducted multiple clinical trials of various G-CSF biosimilars in healthy volunteers and patients with cancer, demonstrating their equivalency to originator compounds. Overall, investigators did not report any significant differences in efficacy, PK/PK, or safety relative to the reference compounds.
A phase III randomized trial evaluated filgrastim and filgrastim-sndz in patients with breast cancer (N = 218) who received G-CSF for CIN prevention. Investigators did not see any clinically significant differences in efficacy (i.e., ANC recovery, FN, hospitalizations, infections) or safety.38 Combined analysis of data from this study and another phase III trial showed a safety profile similar to the originator.39 Retrospective claims analyses and chart reviews also indicated comparable efficacy for this biosimilar and the originator for prophylaxis of neutropenia.40,41,42
A phase III randomized clinical trial in patients receiving myelosuppressive chemotherapy for breast cancer (N = 184), showed that filgrastim-aafi was equivalent in efficacy and safety to the originator product.43 A prospective trial of filgrastim-aafi in 386 patients with solid tumors or hematologic malignancies showed good efficacy and safety results44 that were supported by additional clinical studies.45,46 Similarly, a trial of healthy volunteers showed pegfilgrastim-bmez to have immunogenicity, PK/PD, and safety comparable to its originator product.47 Two randomized clinical trials also found no differences in efficacy (i.e., duration of severe neutropenia) or safety for this biosimilar compared to the originator in patients receiving chemotherapy for breast cancer.48,49 Researchers demonstrated the G-CSF biosimilar pegfilgrastim-apgf to be equivalent to filgrastim at preventing severe neutropenia in women receiving chemotherapy for metastatic breast cancer.50 They also found pegfilgrastim-apgf was more effective than placebo at reducing FN incidence in patients with advanced breast cancer.50 Less published data are available for other G-CSF biosimilars (e.g., pegfilgrastim-cbqv, pegfilgrastim-jmdb). Studies have generally found these drugs to be similar to the reference product with respect to efficacy, PK/PD, and safety.51,52,53,54,55
FDA initially approved tbo-filgrastim through an original biologic license application and subsequently approved it for reduction in the duration of severe neutropenia associated with myelosuppressive chemotherapy. Investigators found tbo-filgrastim to be bioequivalent to filgrastim originator in healthy volunteers.56 Clinical trials of tbo-filgrastim in patients with breast cancer, lung cancer, and non-Hodgkin lymphoma also produced similar outcomes to the reference drug.57,58,59 A meta-analysis of these studies confirmed their findings.60
Safety
G-CSF biosimilars’ safety profiles are generally similar to those of their originator compounds. Bone pain and extremity pain are common adverse events with both G-CSF and its biosimilars (10% to 30% incidence). Patients have reported splenic rupture (possibly fatal), acute respiratory distress syndrome, fatal sickle cell crises, and glomerulonephritis, but all these adverse effects are rare (<2% incidence). Alveolar hemorrhage, hemoptysis, and allergic reactions to G-CSF involving the skin, cardiovascular system, or respiratory system are also possible.14 G-CSF biosimilar use is contraindicated in patients with a history of serious allergic reactions to G-CSF.
In theory, minor differences in manufacturing and processing could alter biosimilars’ immunogenicity.15 However, G-CSF biosimilars appear to lack significant immunogenicity, and pharmaceutical manufacturers are required to submit immunogenicity data to FDA prior to approval. Numerous clinical trials and a systematic review of G-CSF biosimilars found no increase in G-CSF biosimilar immunogenicity compared to the originator compound.28,29,47,55,61,62 In the EU, where biosimilar use is more widespread, regulatory agencies have not raised new concerns regarding immunogenicity. Apart from potential immunogenicity, a pooled analysis of 5 post-approval studies confirmed that a G-CSF biosimilar’s adverse effect profile was consistent with the originator’s, supporting these products’ overall safety.63
Postmarketing pharmacovigilance surveillance programs in larger, more heterogenous populations could be useful to monitor for future safety issues with G-CSF biosimilars that could arise from minor manufacturing differences. According to ASCO, “Sustained development of postmarketing evidence is necessary to enhance patient and provider confidence in biosimilars and to supplement the evidence that supports the safe and effective use of biosimilar products.”15 Accurate nomenclature can help healthcare professionals differentiate among related G-CSF biosimilars and better identify any potential safety issues with a given product.
Clinical Adoption
Overall, the rate of oncology biosimilar approval and clinical adoption has been slower in the U.S. compared with the EU. The reasons for this difference are multifactorial (see Table 4).23 A survey of U.S. physicians indicated that for 74% of respondents, physician confidence was largest barrier to widespread biosimilar adoption, followed by payers (58%), patient education (30%), health policy (27%), and litigation (19%).64 Providers and patients may also be unaware of possible cost savings with biosimilars and the potential for greater patient access biosimilars may provide. While oncology biosimilars offer many advantages, there are certain circumstances that preclude their use, such as when clinical trial protocols or specialty or mail order pharmacy payer preferences dictate originator use.65
| Table 4. Potential Barriers to Wider Clinical Adoption of G-CSF Biosimilars23 |
- Insufficient provider education about biosimilars and similarities/differences compared with originator compounds
|
- Inadequate provider experience with biosimilars
|
- Concerns about equivalency and interchangeability (e.g., efficacy, safety, chemistry, PK/PD)
|
- Extrapolation of approved uses to nonapproved indications
|
- Lack of appreciation of potential benefits (e.g., cost savings, greater patient access)
|
- Concerns about coverage/reimbursement
|
- Patient education and acceptance
|
- Ability to switch to biosimilar (or back to originator) during treatment
|
|
- Rebate programs for originator compounds
|
- Availability of pharmaceutical financial assistance programs
|
| Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics. |
By some measures, though, G-CSF biosimilar adoption in the U.S. has been rapid. An Medicare Part B claims analysis between 2014 and 2016 found a significant decrease in filgrastim originator use (13% lower monthly claims), with increases in filgrastim biosimilars as they became available (Figure 2).66 This analysis included beneficiaries who previously received the originator product and subsequently switched to a G-CSF biosimilar. Interestingly, the prevalence of indications for tbo-filgrastim use was similar to that of the originator, despite the biosimilar having only 1 approved indication. This may reflect clinicians’ familiarity with G-CSF, accepted practices, or comfort level with off-label use for established drugs. A separate retrospective study analyzed claims for commercially insured U.S. patients with cancer who were treated with chemotherapy and G-CSF.42 Filgrastim biosimilar use rose from 7% of users in 2014 to 16% in 2015 and 36% in 2016. Investigators noted no differences in efficacy or safety compared to the originator compound, suggesting filgrastim biosimilar outcomes are comparable to those of the reference compound in the real-world setting.
Figure 2. Filgrastim Medicare Part B Claims, 2014–201666
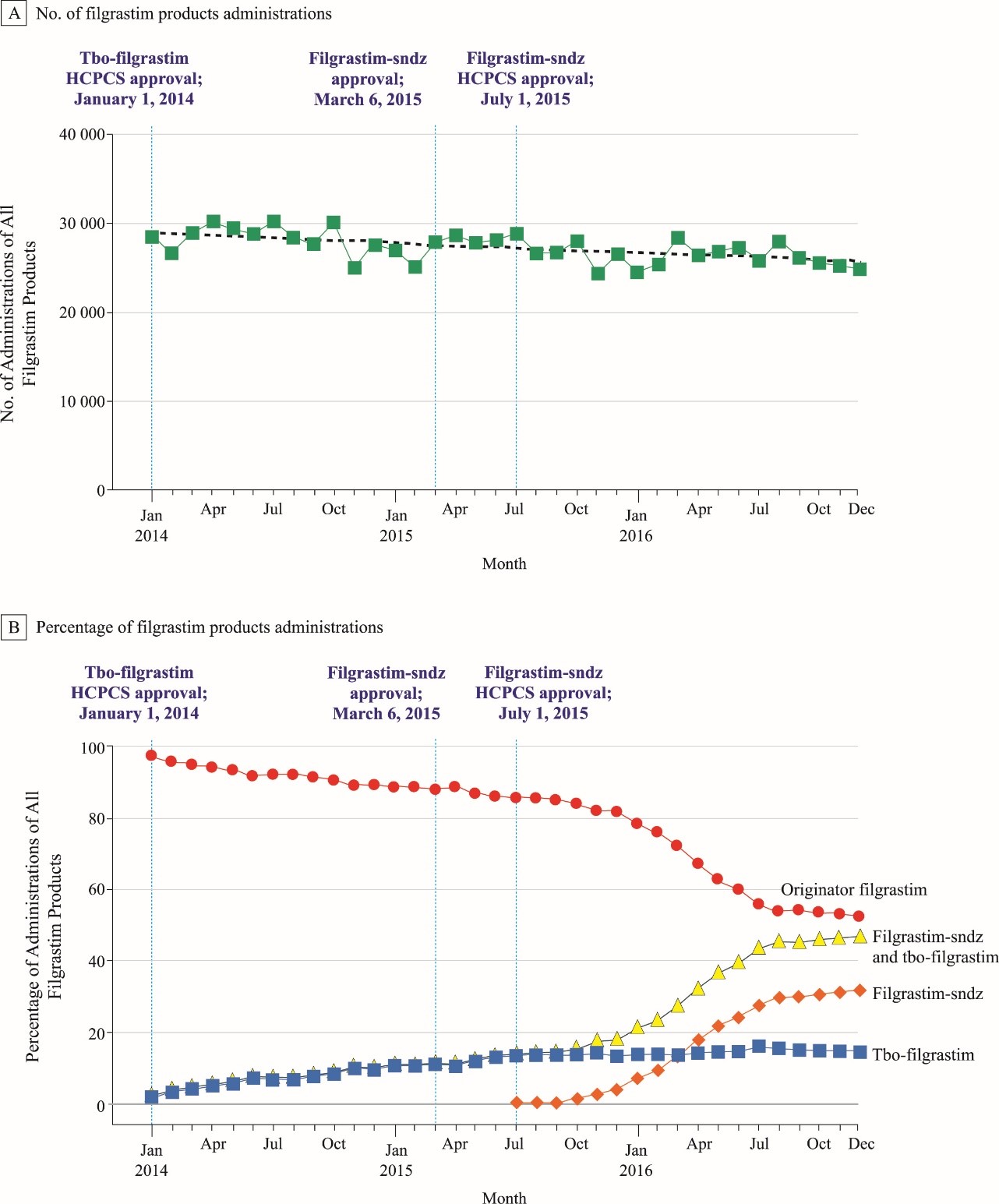
Abbreviation: HCPCS, Healthcare Common Procedure Coding System.
[Reproduced from: Kozlowski S, Birger N, Brereton S, et al. Uptake of the biologic filgrastim and its
biosimilar product among the Medicare population. JAMA. 2018;320(9):929-931.]66
Pharmacoeconomic Considerations
Oncology biologics have significantly impacted clinical outcomes, but the cost of some of these agents can be limiting, especially since many must be used long term. cost of oncology biologics market is rapidly increasing and is projected to have reached nearly $112 billion in the U.S. in 2020.67 Reference compound substitution with biosimilars, therefore, has the potential to realize substantial cost savings. Oncology biosimilars are expected to comprise an increasingly large and important market share since many biologics will soon lose patent exclusivity. It is anticipated that biosimilars will enter the market at up to 30% lower cost compared with their originators, which will encourage price competition.68 In the EU, areas with a high market penetration for biosimilars have realized substantial cost savings.8 The U.S. has not yet achieved such savings, although estimated potential savings from 2017 to 2026 could total $54 billion.69 Increased familiarity and acceptance of biosimilars by healthcare professionals in the U.S. should drive their use and increase cost savings.
Pharmacoeconomic modeling suggests that biosimilar use for a widely-used supportive therapy like G-CSF could result in substantial cost savings to the healthcare system. A German health economic analysis concluded that filgrastim biosimilar use for neutropenia prevention would save the country’s healthcare system € 40.5 million (over $46.5 million U.S. dollars [USD]) annually. They also estimated that pegfilgrastim would save € 56.39 million ($64.5 million USD) annually.70 McBride et al modeled 20,000 patients with non-Hodgkin lymphoma receiving G-CSF prophylaxis for CIN/FN. They estimated that pegfilgrastim-cbqv use would result in a cost savings of $371,444 to $22,286,640, depending on the number of cycles administered and conversion rate.71 A case study of a woman who received dose-intensified epirubicin/paclitaxel/cyclophosphamide for advanced breast cancer projected potential cost savings on an individual patient level. Compared to reference compounds, the potential cost savings for filgrastim were € 5,093 ($6,185 USD) over 9 cycles of chemotherapy, and € 4,199 ($5,100 USD) for pegfilgrastim.71 Cost savings realized by G-CSF biosimilar use might also increase patient access to costly therapies and perhaps help payers provide increased drug access. In general, the availability of multiple G-CSF biosimilars should drive competition, which, assuming equivalent efficacy and safety, will serve to lower drug costs in the marketplace.
Reimbursement/Coverage
Given the high price of many new cancer therapies, drug costs and reimbursement/copays will impact treatment decisions regarding biosimilar use. Biosimilars’ actual value to patients will depend in part on their health plan and related formulary decisions, so specific coverage and reimbursement will vary by payer and patient.15
According to the Centers for Medicare and Medicaid Services, many biosimilars and other biologics are reimbursed under medical rather than pharmacy benefits.15 Medicare considers biosimilars as a “single source” and designates them by a unique Healthcare Common Procedure Code, allowing each to be reimbursed at a separate rate.72 In general, biosimilars are reimbursed at 6% over the average sales price (ASP) less any mandated sequester adjustment; this should incentivize providers to increase biosimilar prescribing. With Medicaid, biosimilar introduction has led to greater competition and to discounts of 20% to 35% relative to reference product prices. Under Medicare Part D, however, biosimilars are exempt from Medicare Coverage Gap Discount Program, resulting in higher out-of-pocket (OOP) costs for some covered individuals.
Pharmaceutical manufacturers may provide patient assistance programs and reimbursement assistance for specialty therapies that could help defray biosimilar costs for some patients, and such programs could vary by manufacturer.73
Pharmacists can help patients navigate insurance coverage and copays for biosimilars and state regulations regarding notification of substitutions and copay or coinsurance differences between a biosimilar and its reference product. They may also assist with benefits verification and prior authorizations to ensure seamless transitions. Pharmacists can facilitate support for insurance or financial issues, such as possible copay or coinsurance differences and patient assistance programs that could help with drug costs.
Pegfilgrastim Biosimilar Versus Pegfilgrastim Autoinjector
Treatment guidelines for FN prophylaxis recommend , one of the following14,15:
- daily injections of filgrastim or a filgrastim biosimilar
- a single injection of pegfilgrastim/biosimilar (due to their longer half-life)
- pegfilgrastim administered through an on-body injector device (PEG-OBI)
Providers usually administer pegfilgrastim 27 hours after infusion of myelosuppressive chemotherapy to decrease effects on the hematopoietic response to G-CSF.
Clinicians can administer pegfilgrastim injections on the same day as chemotherapy to avoid the need for patient return visits, although insurance may not cover such-off label use. A literature review found that many patients with cancer who received prophylactic pegfilgrastim on the same day as chemotherapy had acceptable outcomes. This suggests that this may be a reasonable alternative if a patient is unable to return to the clinic following chemotherapy or wishes to reduce potential COVID-19 exposure.74 PEG-OBI use allows for home administration of pegfilgrastim on the following day, obviating the need for return office visits and enhancing patient convenience.75 (This also applies for Medicare patients, for whom PEG-OBI is covered.) Currently, however, PEG-OBI is approved only for use with originator pegfilgrastim, not its biosimilars.
Researchers have debated the advantages and disadvantages of PEG-OBI versus pegfilgrastim injection. In a study of healthy volunteers, manual injection using a prefilled syringe and on-body injector resulted in comparable pegfilgrastim PK and safety.76 One real-world study found no significant differences in the rates of neutropenia, severe neutropenia, or neutropenic fever in breast cancer patients receiving pegfilgrastim versus PEG-OBI.77 Some data suggest that acceptance and use of PEG-OBI by cancer patients may vary by ethnic group. In 1 study, 22% of patients (87% of whom were Asian) refused PEG-OBI citing the following reasons78:
- bulky attachment
- request to place on stomach instead of arm
- concern over unwitnessed drug administration
- fear of reaction
- disposal at home
- fear of pain
- lack of confirmation of proper dose administration
- the need for MRI
Additionally, PEG-OBI failure rates up to 6.9% have been reported, putting patients at greater risk for FN and FN-related hospitalization. These results highlight the need for better provider–patient communication and patient education, possibly targeting specific ethnic groups, to optimize PEG-OBI use.
Cost modeling studies suggest the potential for significant savings with the use of injected pegfilgrastim biosimilar instead of PEG-OBI. A cost simulation modeled 20,000 patients with lung cancer or non-Hodgkin lymphoma who received G-CSF prophylaxis for FN by daily filgrastim or filgrastim-sndz injections, a single pegfilgrastim injection, or PEG-OBI. Total incremental cost of PEG-OBI was higher than other methods, resulting from incremental costs of FN-related hospitalization due to PEG-OBI failure in cycle 1. The incremental cost amount depended on the estimated PEG-OBI failure rate and alternative G-CSF prophylaxis options.75 Another simulation model studied 15,000 patients with diffuse large B-cell lymphoma at risk for CIN/FN who received PEG-OBI or pegfilgrastim-jmdb. Conversion from PEG-OBI to pegfilgrastim-jmdb realized a total savings of $379,230 to $22,753,800 for cost of medication plus administration, depending on the number of cycles and conversion rate. Such cost savings could allow for expanded access to additional cancer therapy and CIN/FN prophylaxis while reducing risk and hospitalization costs associated with inadequate prophylaxis due to device failure.
Patient OOP costs for PEG-OBI may also be higher than pegfilgrastim injection, depending on insurance coverage. A separate cost-efficiency analysis estimated G-CSF prophylaxis cost for 1 patient during 1 cycle of chemotherapy for non-Hodgkin lymphoma, based on the blended ASP/wholesale acquisition cost rate. Filgrastim-sndz use resulted in significant cost savings compared with filgrastim, pegfilgrastim, or PEG-OBI across various administration scenarios. Over 6 cycles of biosimilar prophylaxis, such savings could provide between 1,690 (at a 10% conversion rate) and 16,900 (100% conversion rate) additional R-CHOP chemotherapy cycles.79
PRESCRIBER AND PATIENT EDUCATION
Prescribers
Despite the number of oncology biosimilars currently marketed in the U.S. (including several for G-CSF), significant knowledge gaps remain for both prescribers and patients. Further education on G-CSF biosimilars and their appropriate use should increase their knowledge and comfort level and lead to wider implementation of these agents.
A 2019 survey assessed oncologists’ knowledge and prescribing patterns for 4 oncology drugs (bevacizumab, trastuzumab, rituximab, and pegfilgrastim) and their biosimilars.80 More than 70% of respondents indicated that biosimilars were the same or nearly equivalent to originators with respect to quality, efficacy, and safety. Notably, the 3 leading drivers for oncology biosimilar use were patient OOP cost, reimbursement value, and cost to the practice. A recent survey of U.S. oncologists, oncology pharmacists, and advanced practice providers, however, found a low comprehension level about biosimilars. Three-quarters of respondents could not adequately define biosimilars, and 40% believed they were the same as generics.67 Moreover, 40% thought it was only somewhat or not important to share decision making about biosimilar use with their patients. The International Society of Oncology Pharmacy Practitioners surveyed its members and national oncology pharmacy groups to identify knowledge gaps on this topic. The top 3 educational needs identified were comparative efficacy of a biosimilar to its originator (74.4%), managing the switch between a biosimilar and originator (74.4%), and understanding related safety issues (73.3%).81 These surveys reveal the need for additional educational resources to better train oncology practitioners on biosimilars, especially as more agents are approved and marketed.
Many educational resources on oncology biosimilars are now available for providers including professional society guidelines and position papers; published medical literature; congresses and seminars; online resources; and material from biosimilar manufacturers (Table 5). These are designed to provide education and guidance on biosimilar nomenclature, regulatory issues, safety and efficacy, interchangeability, switching/substitution, and value.
| Table 5. Educational Resources on Biosimilars |
| Title |
Description |
Source |
| Prescriber Resources |
| ASCO Statement on Biosimilars in Oncology (2018)15 |
Guidance on biosimilar nomenclature, safety, efficacy, interchangeability, and education |
https://ascopubs.org/doi/10.1200/JCO.2017.77.4893?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed |
| FDA Guidance for Industry: Nonproprietary Naming of Biological Products (2017)20 |
FDA recommendations on naming of originator biological products and biosimilars |
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nonproprietary-naming-biological-products-guidance-industry |
| FDA Guidance for Industry: Biosimilarity and Interchangeability: Additional Draft Q&As on Biosimilar Development and the BPCI Act (2020)30 |
FDA draft recommendations and guidance on biosimilar development, interchangeability, and interpretation of the BPCIA |
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/biosimilarity-and-interchangeability-additional-draft-qas-biosimilar-development-and-bpci-act |
| FDA Drug Topics: Biosimilar and Interchangeable Biological Products |
Webinars, slides, and other professional resources on biosimilar science and regulatory process |
https://www.fda.gov/about-fda/fda-pharmacy-student-experiential-program/biosimilar-and-interchangeable-biological-products-updated-review-scientific-concepts-and-practical |
| NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hematopoietic Growth Factors. Version 2.2020 — January 27, 2020.14 |
Current NCCN guidelines on use of G-CSF and other hematopoietic growth factors for prophylaxis and treatment |
www.nccn.org/ |
| Considerations for Use of Hematopoietic Growth Factors in Patients With Cancer Related to the COVID-19 Pandemic (2020)88 |
Guidelines for use of G-CSF and other hematopoietic growth factors in high-risk patients in the context of pandemic SARS-CoV-2 infection |
https://jnccn.org/view/journals/jnccn/aop/article-10.6004-jnccn.2020.7610/article-10.6004-jnccn.2020.7610.xml |
| Waller and Friganovic (2020)21 |
Nursing review of oncology biosimilars |
https://www.futuremedicine.com/doi/full/10.2217/fon-2020-0486?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org |
| AJMC Center for Biosimilars® |
Professional resources and news on biosimilars |
www.centerforbiosimilars.com/ |
| Patient Resources |
| Biosimilar Basics for Patients |
Patient materials on biosimilars and how they compare with biologics and generics |
www.fda.gov/drugs/biosimilars/patient-materials |
| Biosimilar Basics for Patients: What Patients Should Know About Biologics and Biosimilars |
Overview of biosimilars and frequently asked questions |
www.pharmacist.com/ |
| Health Care Provider Materials |
Videos on biosimilars, FDA review process, interchangeability, and other topics |
www.fda.gov/drugs/biosimilars/health-care-provider-materials |
| Drugs.com: Filgrastim |
Patient information on filgrastim originator and biosimilars |
www.drugs.com/mtm/filgrastim.html |
| Drugs.com: Pegfilgrastim |
Patient information on pegfilgrastim originator and biosimilars |
www.drugs.com/mtm/pegfilgrastim.html |
| Abbreviations: AJMC, American Journal of Managed Care; ASCO, American Society of Clinical Oncology; BPCIA, Biologics Price Competition and Innovation Act; COVID-19, coronavirus disease-19; FDA, U.S. Food and Drug Administration; G-CSF, granulocyte-colony stimulating factor; NCCN, National Comprehensive Cancer Network. |
In 2018, ASCO issued a statement emphasizing the need for education on biosimilars for all providers who care for patients with cancer. According to their position, “Continuous provider education is critical to inform, promote, and use biosimilar products in a medically appropriate and cost-effective way to treat cancer.”15 This should include information on prescribing biosimilars and their appropriate dispensing and administration. In addition to oncologists and oncology pharmacists and nurses, education on biosimilars is also indicated for primary care physicians and any other providers involved in cancer care.73
Patients
In addition to professional education, patient education is also needed to facilitate G-CSF biosimilar implementation. Patient education on biosimilars from trusted medical sources and practitioners will improve patients’ understanding of these products and increase their confidence regarding safety and efficacy. Many patients with cancer believe that less-expensive drugs are as effective as more costly ones and would be willing to have their physicians prescribe lower-cost option.82 Yet a recent study of patients with autoimmune disorders reported that 85% were concerned about side effects if they were switched to a biosimilar for a nonmedical reason.83 Leading reasons for their reluctance to switch included physician recommendations, expected efficacy and safety, and OOP cost. Patients also may experience a “nocebo effect” when switching from an originator to a biosimilar. This is when a patient perceives a negative symptom that is not drug induced but instead is based on their negative expectations/education or by negative healthcare professional suggestions.84 These results highlight the importance of good communication between providers and patients and the need for better patient education regarding biosimilar use. Increased healthcare professionals’ use of and confidence in prescribing G-CSF biosimilars should result in greater patient acceptance of these drugs.
Oncologists and other healthcare professionals should provide supplemental patient educational materials to help explain biosimilars and their use, safety, and interchangeability. These resources should be tailored to an appropriate reading level and specific demographics/languages to ensure patient understanding and acceptance. Oncologists and oncology nurses, nurse practitioners, and pharmacists can serve as key resources for patient information regarding treatment with G-CSF biosimilars.15,85,86,87 In addition to existing online resources (see Table 5), social media, patient advocacy groups, and biosimilar manufacturers might also provide patient education programs. Pharmacovigilance programs for G-CSF biosimilars would also help reassure patients about their safety.
CONCLUSION
Surveys of healthcare professionals indicate that although knowledge and acceptance of oncology biosimilars is growing, significant gaps remain, highlighting the need for further education.
The rising number of marketed biosimilars, and G-CSF biosimilars in particular, requires practitioners to be familiar with their characteristics, approved indications, and best practices to ensure optimal use. In addition, they must also be aware of state regulations pertaining to substitution and interchangeability of biosimilars with their originators.
The full potential of G-CSF biosimilars has not yet been fully realized in this country. Increasing G-CSF biosimilar use should result in greater comfort with and acceptance of these agents by healthcare professionals and patients. As more G-CSF biosimilars enter the U.S. market, competition could result in price reductions, mitigating overall healthcare expenditures and disparities in access to cancer care. These agents thus represent an important alternative treatment that could benefit individual patients and public healthcare in general.
During the COVID-19 pandemic, patients with cancer face an increased SARS-CoV-2 infection risk due to their immunocompromised state and the continuing need for provider visits to receive cancer therapy. Greater use of G-CSF and its biosimilars could help lower the risks of CIN, FN, and infection, thus decreasing the frequency of visits to hospitals or outpatient settings and reducing potential exposure. Recent NCCN guidelines support expanded prophylactic G-CSF use in these patients, and increased use of G-CSF to accelerate recovery of ANC following hematopoietic cell transplantation.88 The guidelines also suggest that patients consider self-administration of daily filgrastim or long-acting pegfilgrastim use for the same reasons. Expanded G-CSF biosimilar use in these situations may be indicated.
Oncology pharmacists and nurses can help educate and influence their professional colleagues regarding G-CSF biosimilar use, which will encourage more widespread utilization of these agents in routine clinical practice. Both pharmacists and nurses should work in conjunction with clinicians in the decision-making process when selecting a G-CSF–based therapy. They also can aid in safety and adherence monitoring as these products are more widely used. The availability of additional safety and efficacy data could alleviate concerns some prescribers may have regarding G-CSF biosimilar use. Further patient and caregiver education will help alleviate any apprehension they may have regarding their efficacy and safety.
References
- Park J, Look KA. Health care expenditure burden of cancer care in the United States. Inquiry. 2019;56:1-9.
- Villanueva MN, Davis JE, Sobocinski SM. Navigating uncharted waters: Developing a standardized approach for evaluating and implementing biosimilar products at a comprehensive cancer center. Am J Health Syst Pharm. 2021;78(3):249-260.
- Shah B, Palinkas N. Current and future considerations for oncology biosimilars in 2020. Accessed at https://www.pharmacytimes.com/publications/Directions-in-Pharmacy/2019/December2019/featured-articles-current-and-future-considerations-for-oncology-biosimilars-in-2020, April 16, 2021.
- Huang HY, Wu DW, Ma F, et al. Availability of anticancer biosimilars in 40 countries. Lancet Oncol. 2020;21(2):197-201.
- Generics and Biosimilars Initiative (GaBI). Approval and launch dates for US biosimilars. Accessed at https://www.gabionline.net/Reports/Approval-and-launch-dates-for-US-biosimilars, April 16, 2021.
- U.S. Food & Drug Administration. Biosimilar product information. Accessed at https://www.fda.gov/drugs/biosimilars/biosimilar-product-information, April 16, 2021.
- Saleem T, Qurashi H, Jamali M, et al. Biosimilars as a future, promising solution for financial toxicity: a review with emphasis on bevacizumab. Cureus. 2020;12(7):e9300.
- Quintiles IMS. The impact of biosimilar competition in Europe. London: May 2017. Accessed at https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf, April 16, 2021.
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228-237.
- Awad M, Singh P, Hilas O. Zarxio (filgrastim-sndz): the first biosimilar approved by the FDA. P T. 2017;42(1):19-23.
- Griffith N, McBride A, Stevenson JG, et al. Formulary selection criteria for biosimilars: considerations for US health-system pharmacists. Hosp Pharm. 2014;49(9):813-825.
- Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42(15):2433-2453.
- Vanderpuye-Orgle J, Sexton Ward A, Huber C, et al. Estimating the social value of G-CSF therapies in the United States. Am J Manag Care. 2016;22(10):e343-e349.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hematopoietic Growth Factors. Version 2.2020 — January 27, 2020.
- Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: Biosimilars in oncology. J Clin Oncol. 2018;36(12):1260-1265.
- NCCN Hematopoietic Growth Factors. Short-term recommendations specific to issues with COVID-19 (SARS-CoV-2). Accessed at https://www.nccn.org/covid-19/pdf/HGF_COVID-19.pdf, April 16, 2021.
- U.S. Department of Health and Human Services. Scientific considerations in demonstrating biosimilarity to a reference product: Guidance for industry. April 2015. Accessed at https://www.fda.gov/media/82647/download, April 16, 2021.
- U.S. Food and Drug Administration. Generic drug facts. Updated June 1, 2018. Accessed at www.fda.gov/drugs/generic-drugs/generic-drug-facts, April 16, 2021.
- Harvey RD. Science of biosimilars. J Oncol Pract. 2017;13(9_suppl):17s-23s.
- U.S. Department of Health and Human Services. Nonproprietary naming of biological products: Guidance for industry. Accessed at https://www.fda.gov/media/93218/download, April 16, 2021.
- Waller CF, Friganović A. Biosimilars in oncology: key role of nurses in patient education. Future Oncol. 2020;16(25):1931-1939. Accessed at https://www.futuremedicine.com/doi/full/10.2217/fon-2020-0486?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org, February 21, 2021.
- U.S. Food and Drug Administration. Biosimilars. Accessed at https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars, April 16, 2021.
- Whalen J. Recognizing and addressing challenges to the adoption of trastuzumab biosimilars and HER2-targeted therapies. Am J Manag Care. 2020;26(2 Suppl):S23-S31.
- Zelenetz AD. The era of therapeutic biosimilars has arrived: what you need to know. J Natl Compr Canc Netw. 2019;17(11.5):1424-1426.
- Duncan G, Willoughby R. Second medical use claims and 'skinny' labels: clear guidance at last? Pharm Pat Anal. 2016;5(3):137-139.
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40(10):680-689.
- Cauchi R. State laws and legislation related to biologic medications and substitution of biosimilars. National Conference of State Legislatures. May 3, 2019. Accessed at https://www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx, April 16, 2021.
- Blackwell K, Gascon P, Krendyukov A, et al. Safety and efficacy of alternating treatment with EP2006, a filgrastim biosimilar, and reference filgrastim: a phase III, randomised, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2018;29(1):244-249.
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463-478.
- U.S. Food and Drug Administration. Biosimilarity and interchangeability: additional draft Q&As on biosimilar development and the BPCI Act. November 2020. Accessed at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/biosimilarity-and-interchangeability-additional-draft-qas-biosimilar-development-and-bpci-act, April 16, 2021.
- Cornes P, Gascon P, Chan S, et al. Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv Ther. 2018;35(11):1816-1829.
- Blair HA, Scott LJ. Tbo-filgrastim: a review in neutropenic conditions. BioDrugs. 2016;30(2):153-160.
- Konstantinidou S, Papaspiliou A, Kokkotou E. Current and future roles of biosimilars in oncology practice. Oncol Lett. 2020;19(1):45-51.
- Neulasta [package insert]. Thousand Oaks, CA: Amgen Inc.;2021.
- Neupogen [package insert]. Thousand Oaks, CA: Amgen Inc.;2021.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199-3212.
- Lazarus HM, Gale RP. G-CSF and GM-CSF are different. Which one is better for COVID-19? [published online ahead of print, 2020 Aug 13]. Acta Haematol. 2020;1-4. doi:10.1159/000510352
- Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948-1953.
- Blackwell K, Gascon P, Jones CM, et al. Pooled analysis of two randomized, double-blind trials comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Ann Oncol. 2017;28(9):2272-2277.
- Zecchini J, Yum K, Steinberg A, et al. A single-center, retrospective analysis to compare the efficacy and safety of filgrastim-sndz to filgrastim for prophylaxis of chemotherapy-induced neutropenia and for neutrophil recovery following autologous stem cell transplantation. Support Care Cancer. 2018;26(3):1013-1016.
- Schwartzberg LS, Lal LS, Balu S, et al. Clinical outcomes of treatment with filgrastim versus a filgrastim biosimilar and febrile neutropenia-associated costs among patients with nonmyeloid cancer undergoing chemotherapy. J Manag Care Spec Pharm. 2018;24(10):976-984.
- Chen X, Agiro A, Barron J, et al. Early adoption of biosimilar growth factors in supportive cancer care. JAMA Oncol. 2018;4(12):1779-1781.
- Waller CF, Semiglazov VF, Tjulandin S, et al. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504-511.
- Fruehauf S, Otremba B, Stotzer O, Rudolph C. Compatibility of biosimilar filgrastim with cytotoxic chemotherapy during the treatment of malignant diseases (VENICE): a prospective, multicenter, noninterventional, longitudinal study. Adv Ther. 2016;33(11):1983-2000.
- Frederic M, Stephane L, Didier K, et al. Biosimilar filgrastim in the treatment and the prevention of chemotherapy-induced neutropenia: The next study. J Geriatr Oncol. 2014;5(Suppl 2):S71 (abstract).
- Brito M, Esteves S, Andre R, et al. Comparison of effectiveness of biosimilar filgrastim (Nivestim), reference Amgen filgrastim and pegfilgrastim in febrile neutropenia primary prevention in breast cancer patients treated with neo(adjuvant) TAC: a non-interventional cohort study. Support Care Cancer. 2016;24(2):597-603.
- Nakov R, Gattu S, Wang J, et al. Proposed biosimilar pegfilgrastim shows similarity in pharmacokinetics and pharmacodynamics to reference pegfilgrastim in healthy subjects. Br J Clin Pharmacol. 2018;84(12):2790-2801.
- Blackwell K, Donskih R, Jones CM, et al. A comparison of proposed biosimilar LA-EP2006 and reference pegfilgrastim for the prevention of neutropenia in patients with early-stage breast cancer receiving myelosuppressive adjuvant or neoadjuvant chemotherapy: pegfilgrastim randomized oncology (supportive care) trial to evaluate comparative treatment (PROTECT-2), a phase III, randomized, double-blind trial. Oncologist. 2016;21(7):789-794.
- Harbeck N, Lipatov O, Frolova M, et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Future Oncol. 2016;12(11):1359-1367.
- Nyvepria [package insert]. New York, NY: Pfizer, Inc.;2020.
- Glaspy JA, O'Connor PG, Tang H, Finck B. Randomized, single-blind, crossover study to assess the pharmacokinetic and pharmacodynamic bioequivalence of CHS-1701 to pegfilgrastim in healthy subjects. J Clin Oncol. 2017;35(Suppl 15):e21693 (abstract).
- O'Connor P, Tang H, Civoli F, et al. Proposed pegfilgrastim biosimilar CHS-1701 demonstrates pharmacokinetic and pharmacodynamic similarity to marketed pegfilgrastim in a rat neutropenia model and in healthy subjects [abstract]. Proceedings of the 22nd Congress of the European Hematology Association 2017. Accessed at https://learningcenter.ehaweb.org/eha/2017/22nd/180923/paula.oconnor.proposed.pegiflgrastim.biosimilar.chs-1701.demonstrates.html, April 16, 2021.
- Waller C, Ranganna GM, Pennella E, et al. Comparison of immunogenicity between the proposed pegfilgrastim biosimilar MYL-1401H and reference pegfilgrastim. Blood. 2017;130(Suppl 1):3568 (abstract).
- Waller CF, Tiessen RG, Lawrence TE, et al. A pharmacokinetics and pharmacodynamics equivalence trial of the proposed pegfilgrastim biosimilar, MYL-1401H, versus reference pegfilgrastim. J Cancer Res Clin Oncol. 2018;144(6):1087-1095.
- Waller CF, Ranganna GM, Pennella EJ, et al. Randomized phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H in the prophylactic treatment of chemotherapy-induced neutropenia. Ann Hematol. 2019;98(5):1217-1224.
- Lubenau H, Sveikata A, Gumbrevicius G, et al. Bioequivalence of two recombinant granulocyte colony-stimulating factor products after subcutaneous injection in healthy volunteers. Int J Clin Pharmacol Ther. 2009;47(4):275-282.
- Gatzemeier U, Ciuleanu T, Dediu M, et al. XM02, the first biosimilar GCSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 2009;4(6):736-740.
- del Giglio A, Eniu A, Ganea-Motan D, et al. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332.
- Engert A, Griskevicius L, Zyuzgin Y, et al. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 2009;50(3):374-379.
- Engert A, del Giglio A, Bias P, et al. Incidence of febrile neutropenia and myelotoxicity of chemotherapy: a meta-analysis of biosimilar G-CSF studies in breast cancer, lung cancer, and non-Hodgkin's lymphoma. Onkologie. 2009;32(10):599-604.
- Harbeck N, Gascon P, Krendyukov A, et al. Safety profile of biosimilar filgrastim (Zarzio/Zarxio): a combined analysis of phase III studies. Oncologist. 2018;23(4):403-409.
- Yao HM, Ottery FD, Borema T, et al. PF-06881893 (Nivestym™), a filgrastim biosimilar, versus U.S.-licensed filgrastim reference product (U.S.-Neupogen®): pharmacokinetics, pharmacodynamics, immunogenicity, and safety of single or multiple subcutaneous doses in healthy volunteers. BioDrugs. 2019;33(2):207-220.
- Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925-2932.
- AmerisourceBergen. Biosimilars check up: physician perspectives on utilization, cost and barriers to adoption. September 10, 2019. Accessed at https://www.amerisourcebergen.com/insights/manufacturers/biosimilar-physician-insights-survey, April 16, 2021.
- Cuellar S, McBride A, Medina P. Pharmacist perspectives and considerations for implementation of therapeutic oncology biosimilars in practice. Am J Health Syst Pharm. 2019;76(21):1725-1738.
- Kozlowski S, Birger N, Brereton S, et al. Uptake of the biologic filgrastim and its biosimilar product among the Medicare population. JAMA. 2018;320(9):929-931.
- Cook JW, McGrath MK, Dixon MD, et al. Academic oncology clinicians' understanding of biosimilars and information needed before prescribing. Ther Adv Med Oncol. 2019;11:1-12.
- Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist. 2018;23(11):1261-1265.
- Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3.
- Hübel K, Kron F, Lux MP. Biosimilars in oncology: Effects on economy and therapeutic innovations. Eur J Cancer. 2020;139:10-19.
- McBride A, MacDonald K, Abraham I. Simulation modeling of cost-savings from conversion to biosimilar pegfilgrastim-cbqv for the prophylaxis of chemotherapy-induced neutropenia, and budget-neutral expanded access to prophylaxis and anti-neoplastic therapy from derived cost-savings in non-Hodgkin lymphoma. Presented at American Society of Hematology Annual Meeting, December 5-8, 2020, San Diego, CA (abstr 3425).
- Lyman GH, Zon R, Harvey RD, Schilsky RL. Rationale, opportunities, and reality of biosimilar medications. N Engl J Med. 2018;378(21):2036-2044.
- Simoens S, Jacobs I, Popovian R, et al. Assessing the value of biosimilars: a review of the role of budget impact analysis. Pharmacoeconomics. 2017;35(10):1047-1062.
- Gerberich AJ, Attilio MR, Svoboda A. Revisiting same day administration of pegfilgrastim in the age of biosimilars: A review of literature. J Oncol Pharm Pract. 2020;26(8):1970-1976.
- McBride A, Krendyukov A, Mathieson N, et al. Febrile neutropenia hospitalization due to pegfilgrastim on-body injector failure compared to single-injection pegfilgrastim and daily injections with reference and biosimilar filgrastim: U.S. cost simulation for lung cancer and non-Hodgkin lymphoma. J Med Econ. 2020;23(1):28-36.
- Yang BB, Morrow PK, Wu X, et al. Comparison of pharmacokinetics and safety of pegfilgrastim administered by two delivery methods: on-body injector and manual injection with a prefilled syringe. Cancer Chemother Pharmacol. 2015;75(6):1199-1206.
- Maahs L, Tang A, Saheli ZA, et al. Real-world effectiveness of the pegfilgrastim on-body injector in preventing severe neutropenia [published online ahead of print, 2020 Dec 15]. J Oncol Pharm Pract. doi: 10.1177/1078155220980517.
- Saif MW, Hackenyos DW, Smith MH, et al. Racial differences in accepting pegfilgrastim Onpro kit (on-body injector) use among cancer patients. Clin Oncol (Las Vegas). 2019;1(6):1026.
- McBride A, MacDonald K, Abraham I. Conversion to biosimilar pegfilgrastim-jmdb from pegfilgrastim with on-body injector device in diffuse large B-cell lymphoma: simulation modeling of cost-savings and budget-neutral expanded access to prophylaxis and anti-neoplastic therapy considering device failure rate. Presented at American Society of Hematology Annual Meeting, December 5-8, 2020, San Diego, CA (abstr 3422).
- Galan C, Johnson KA, Avalos-Reyes E. US oncologists' perception of the efficacy, safety, and willingness to prescribe biosimilar cancer therapies. J Clin Oncol. 2020;38(Suppl 15):e15213.
- Chan A, Patel H, Siderov J, et al. Assessing biosimilar education needs among oncology pharmacy practitioners worldwide: An ISOPP membership survey. J Oncol Pharm Pract. 2020;26(Suppl 3):11-21.
- Harvey RD, McGrath M, Cook JW, et al. How will the cost of biosimilars affect patients’ willingness to receive them? J Clin Oncol. 2019;37(Suppl 15):e18338.
- Teeple A, Ginsburg S, Howard L, et al. Patient attitudes about non-medical switching to biosimilars: results from an online patient survey in the United States. Curr Med Res Opin. 2019;35(4):603-609.
- Kristensen LE, Alten R, Puig L, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs. 2018;32(5):397-404.
- Zelenetz AD, Ahmed I, Braud EL, et al. NCCN biosimilars white paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Canc Netw. 2011;9(Suppl 4):S1-S22.
- Hematology/Oncology Pharmacy Association. Biosimilars issue brief: an important new category of medications for cancer patients. Revised December 2015. Accessed at http://www.hoparx.org/images/hopa/advocacy/Issue-Briefs/HOPA_Biosimilars_Issue_Brief.pdf, April 16, 2021.
- Vizgirda V, Jacobs I. Biosimilars: considerations for oncology nurses. Clin J Oncol Nurs. 2017;21(2):E54-E60.
- Griffiths EA, Alwan LM, Bachiashvili K, et al. Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic [published online ahead of print, 2020 Sep 1]. J Natl Compr Canc Netw. 2020:1-4. doi:10.6004/jnccn.2020.7610.
- Zarxio [package insert]. Princeton, NJ: Sandoz Inc;2015.
- Nivestym [package insert]. New York, NY: Pfizer, Inc.;2018.
- Granix [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;2014.
- Ziextenzo [package insert]. Princeton, NJ: Sandoz Inc;2019.
- Udenyca [package insert]. Redwood City, CA: Coherus BioSciences;2018.
- Fulphila [package insert]. Rockford, IL: Mylan Institutional LLC;2018.
Back to Top