
Expired activity
Please go to the PowerPak
homepage and select a course.
TECHNICIAN’S ROLE IN MEDICATION SAFETY
Pharmacy technicians have important responsibilities for taking part in medication safety practices. Pharmacy technicians:
- enter prescription information
- interact with and collect data from patients
- assist with drug dispensing
- keep records, and
- restock medications,
With these duties, pharmacy technicians have the potential to commit errors. However, technicians also have opportunities to avoid or intercept mistakes and to take steps to prevent future errors.
HOW MEDICATION SAFETY PLAYS INTO MTM
Reducing and preventing medication errors is part of the technician’s everyday job. This chapter will focus on medication safety as an important aspect of medication therapy management (MTM). A major goal of MTM is to prevent and resolve errors and safety risks associated with medication use. Pharmacists and pharmacy technicians serve as essential resources in ongoing efforts toward reducing and preventing errors and avoidable safety risks in healthcare delivery.1
MTM is a way to take a closer examination of the medications a patient has been prescribed, and to determine which of these prescription and OTC medications they actually use. Thus the process of MTM is designed to tease out medication errors. Through their roles in gathering and recording patient information during MTM, pharmacy technicians may be in a position to detect and intercept medication errors. Patients who experience medication errors, such as the wrong type or dose of a drug, often lose trust in the process.
Many pharmacy technicians have taken courses or had training in the prevention of medication errors. However, it is always advisable for technicians to update their training because of changes that arise in electronic health record (EHR) systems, new medications, and new internal systems within the pharmacy.
How Much of a Problem is Medication Safety?
The issue of medication errors came into focus with the release of a widely publicized report “To Err is Human: Building a Safer Health System,” published by the Institute of Medicine (IOM).2 Although some experts suggest that medication error rates cited in this document may be exaggerated or flawed, this this report was among the first to focus major attention on the problem. Primary conclusions from “To Err is Human” include:2
- Medical errors cause between thousands of deaths in American hospitals each year, and many more injuries that do not result in death.
- The majority of medical errors do not result from individual recklessness or carelessness, but are caused by faulty systems, processes, and conditions that increase the chances for mistakes to occur.
- When an error occurs, blaming an individual does not make the system any safer, or prevent another person from committing the same error. Instead of blaming individuals, systems should be examined and improved to prevent errors.
Other reports related to medication errors have shown:
- Serious medication errors occur in 5% to 10% of patients admitted to hospitals;3
- Each year, in the United States alone, 7,000 to 9,000 people die as a result of a medication error;4
- Each year, an estimated 1.5 million preventable adverse drug events occur, costing up to $177 billion due to patient injury and death;3
- Adverse drug events cause more than 770,000 injuries and deaths each year and cost up to $5.6 million in each hospital;5
- Efforts to reduce preventable errors have resulted in a significant drop in drug–related adverse events in recent years.6
In 2015, the IOM changed its name to the National Academy of Medicine. Since its year 2000 report, this organization has released many other documents and studies related to medication safety. Another important organization focusing on medication error prevention is the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP), an independent body composed of 27 national healthcare organizations.7
WHAT EXACTLY IS A MEDICATION ERROR?
Medication errors can be very broadly defined as: “Any error occurring in the medication use process.”
The NCC MERP definition of a medication error is:
“Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer.”7 Such events may be related to:
- Professional practice healthcare products, procedures, and systems, including prescribing
- Order communication
- Product labeling, packaging, and nomenclature
- Compounding; dispensing; distribution
- Administration; education; monitoring; and use
The IOM defines a medication error as “A breakdown or failure at any point in the medication use process—from prescribing, to use by the patient.”1 This may include errors such as those shown in Figure 1.
| Figure 1. Sources of Medication Errors |
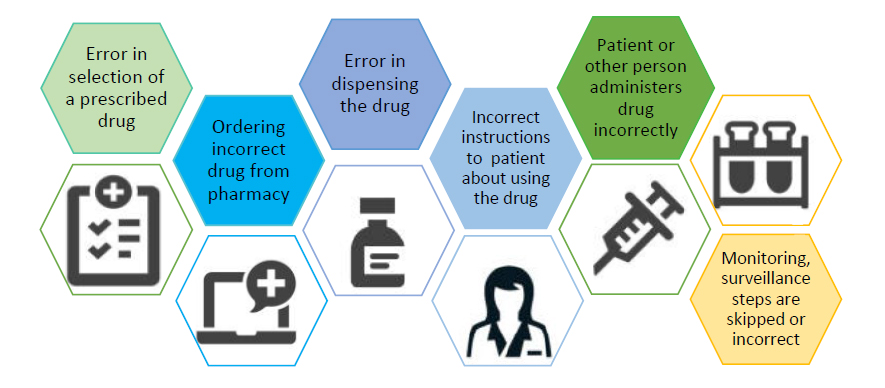 |
An event does not need to cause actual patient harm to be considered an error—it only needs to have the potential to lead to patient harm or inappropriate use of a drug or device. An example might be that an existing drug allergy is overlooked when a patient receives a prescription, but the patient does not experience an allergic reaction when taking it this time. This counts as an error because the problem had the potential to cause harm to the patient, and could have been prevented with improved documentation. Another example might be an error this is made by a health professional, but is caught by the pharmacist or pharmacy technician, patient, or other individual. It is always good to catch or intercept an error after it is made and before it causes harm—but it would be considered an error nonetheless.
TYPES OF MEDICATION ERRORS AND THEIR CAUSES
Common types of medication errors are broken down in Figure 2. Of the roles that directly involve pharmacists and pharmacy technicians, prescription order processing is thought to be responsible for about 12% of all medication errors, while preparation and dispensing cause about 11%.8
| Figure 2. Types of Medication Errors |
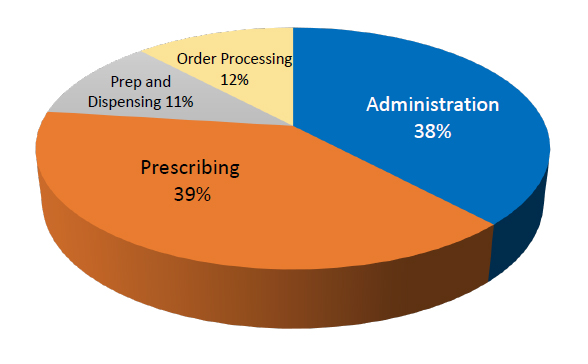 |
| Adapted from: Lisby M, et al. Int J Qual Health Care. 2005;17(1):15-22.8 |
Prescribing errors
Prescribing errors relate to the initial prescription by the physician or other authorized prescriber. These errors are thought to make up about 39% of all medication errors.7 Examples may include:
- Incorrect dosing (e.g., dose adjustment for kidney function is neglected)
- Drug interactions with another prescription taken by the patient
- Illegible handwriting (for handwritten prescriptions)
- Electronic prescribing errors (e.g., wrong medication, wrong strength)
Although these errors are made by the prescriber, there are many ways a pharmacy technician and/or pharmacist may detect and intercept these errors, by verifying the information with the physician when necessary. If the pharmacy technician is in doubt about handwriting or some other aspect of a prescription, it is essential that the situation be reported to the pharmacist, rather than trying to make an educated guess.
Confusing abbreviations and sound–alike drugs contribute to prescribing errors. Technicians should consult the tools offered by the Institute for Safe Medicine Practices (ISMP) for lists of look–alike/sound–alike medications, banned abbreviations, and other resources.9 (See http://www.ismp.org/tools/)
Preparation and dispensing errors
Preparation and dispensing errors occur when an incorrect medication or dose is dispensed to the patient. These errors might occur at the pharmacy level, or, in a hospital, through the use of automated dispensing machines that contain incorrect or missing medications. Some examples include:
- Wrong medication, dose, or dosage form
- Drug delivered or distributed to incorrect person
- Wrong concentration or diluent (compounding)
- Interchanging of immediate vs. extended release products
- Errors of omission/wrong time
- Wrong or missing auxiliary labeling
Omission errors
An omission error is when a prescribed medication is not administered. A “wrong time” error refers to a delay or administration of a medication at the incorrect time.8 For example, a delay may occur because of the need to obtain prior authorization from an insurance company or payer prior to filling the prescription. In some cases, a delay or omission has the potential to have a negative impact on the patient’s health. In order to address these types of errors, pharmacy technicians can help to minimize barriers that prevent patients from receiving prescribed medications. Some steps may include ensuring that medications are properly stocked, that questions for physicians’ offices are handled promptly, and that insurance coverage issues are managed in a timely manner.
Administration errors
Administration errors usually refer to mistakes made by the patient or caregiver in taking a medication. Examples of administration errors may include:
- Patient takes a medication not prescribed for him or her (e.g., a person takes an antibiotic that was previously prescribed for another family member for a different condition)
- Patient uses the wrong medication (mistakes one medication bottle for another, or similar–looking pill for the medication they intended)
- Patient takes the wrong dose of the medication
- Patient omits the medication or takes it at the wrong time
- Wrong route of administration (e.g., intramuscular instead of subcutaneous)
- Not taking a medication as directed (e.g., not taking it on an empty stomach or with a meal).
Even though the patient is involved, these errors may originate at other stages in the drug– delivery process. For example, the patient might have gotten unclear directions, missing instructions, missing or incorrect labeling. The prescriber and pharmacist may have failed to verify with the patient that he or she understands how to take the medication. Physicians often give patients instructions about how many doses to take per day, and how many pills per dose, very quickly, and often cover multiple medications at the same time. If the technician follows protocols to assist patients, many of these errors of misunderstanding can be prevented. Technicians can encourage patients to accept pharmacist counseling, and in some areas of the country this suggestion is required. (Patients may be more likely to accept counseling when the suggestion comes from the technician.)
Knowing the types of behaviors that lead to errors is key to prevention. NCC MERP developed a list of at–risk behaviors relevant to pharmacy technicians, summarized in Table 1.10
Table 1. At-Risk Behaviors for Medication Errors
As defined by National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)10 |
| Category |
Types of Errors |
| I. Patient Information |
• Not checking patient identification using two identifiers (e.g., name, medical record number, birth date)
• Not checking a patient's allergies before prescribing/dispensing/administering medications
• Not viewing/checking the patient's complete medication profile (or medication administration record [MAR]) prior to prescribing/dispensing/administering medications
|
| II. Drug Information |
• Prescribing/dispensing/administering medications without complete knowledge of the medication
• Failure to address computer-driven warnings
• Not questioning unusually large doses of medications
• Failure to visually inspect the medication to be administered
• Failing to validate/reconcile the medications and doses that the patient states are taken at home
|
| III. Communication |
• Rushed communication with next shift/covering colleague
• Intimidation/not speaking up when there is a question or concern about a medication
• Use of error–prone abbreviations/apothecary designations/dangerous dose designations
• Unnecessary use of verbal orders [observed less often]
• Not reading back verbal orders [observed less often]
|
| IV. Labeling, Packaging, Nomenclature |
• Absent or poor labeling of syringes, solutions, and/or other medication packages
• Grab and go without fully reading the label of a medication before dispensing/administering/restocking medications
• Storing medications with look–alike, sound–alike labels and packaging beside one another
|
| V. Drug Stock, Storage, Distribution |
• Leaving medications in an unlocked storage area
• Preparing IV admixtures outside of the pharmacy
|
| VI. Environment/Staffing Patterns |
• Managing multiple priorities while carrying out complex processes (e.g., order entry, transcription, drug administration, IV admixture)
• Holding/admitting overflow patients in inappropriate units/areas
• Failure to adequately supervise/orient staff
• Inadequate staffing based on patient acuity
|
| VII. Patient Education |
• Prescribing/administering/dispensing medications without educating patient
• Disregarding patient's/caregiver’s concerns about a medication's appearance, reactions, side effects, or other expressed worry
• Failure to follow up regarding a medication’s intended effect against the patient’s observed or reported effect
|
| VIII. Staff Education |
• Inadequate orientation of new/agency staff
• No organizational incentives to achieve certification or attend continuing education
• Lack of a structured and ongoing staff competency program related to medication use
|
| IX. Quality/Culture |
• Sacrificing safety for timeliness
• Failure to report and share error information (anonymous reporting improves safety and outcomes)
• Organizational culture of secrecy rather than openness about medication errors
• Organizational culture of finger pointing rather than system change
• Failure to address interruptions during medication ordering, preparation, and administration
• Failure to properly implement and evaluate new technology
|
| X. Double Checks |
• Failure to ask a colleague to double–check manual calculations before proceeding
• Failure to ask a colleague to double–check high–alert medications before dispensing/administration
• Failure to ask a colleague to double–check high–risk processes (e.g., patient–controlled analgesia) before proceeding
|
| XI. Teamwork |
• Reluctance to consult others or ask for help when indicated
• Lack of responsiveness to colleague/patient requests
|
| XII. Technology |
• Technology work–arounds
• Overriding computer alerts without due consideration (alert fatigue)
• Failure to fully engage available technology
|
CAN MEDICATION ERRORS BE PREVENTED?
Errors can never be prevented 100% of the time. However, if enough checks and balances exist—and if errors are reported in an honest and timely manner—the error can be caught before it causes harm. One example of how this might work is illustrated in the “Swiss Cheese” model of error prevention. According to this model, many layers of defense may exist to prevent hazards and accidents, but within each layer there are usually one or more minor flaws or “holes.” If the holes are too frequent, or are aligned with each other, an accident or error is more likely to occur (see Figure 3).11
| Figure 3. Swiss Cheese Model of Error Causation |
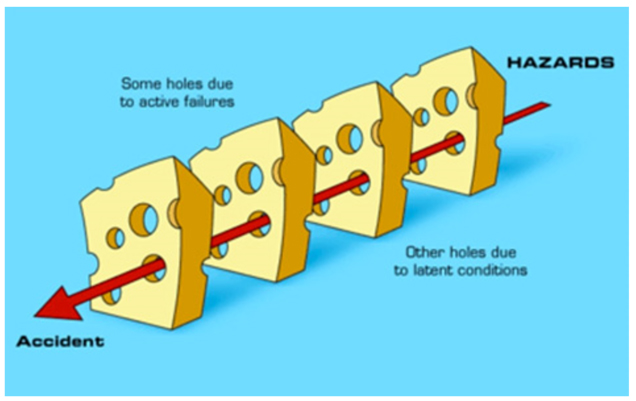 |
| Source: Reason J. Human error: models and management. BMJ. 2000;320(7237):768-770.11 |
An example of how this might relate to a medication error is as follows:
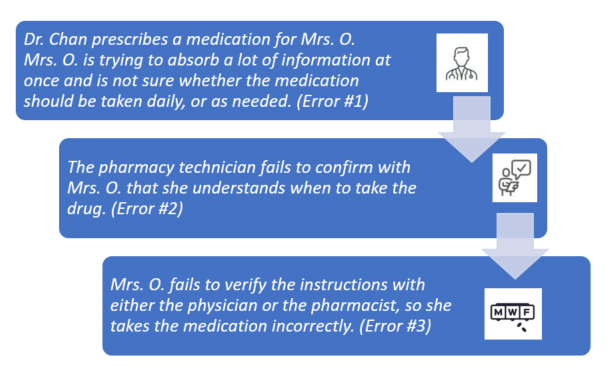
In this scenario, there are several potential places where the error might have been stopped. Because they weren’t, the “holes” or errors lined up to allow Mrs. O. to take the medication incorrectly.
Some tips technicians can apply to prevent errors are summarized in Table 2.
| Table 2. Error Prevention Tips for Pharmacy Technicians |
|
Prescription drop-off
- Create a checklist of key information to be obtained from each patient
- Write date of birth on hard copy Rx to serve as a second identifier
- Update allergy/medical conditions (e.g., pregnancy) at each patient encounter
- Communicate all relevant changes to the pharmacist
Order entry
• Determine methods for updating knowledge about newly approved drugs
• Recognize safety features of the computer system and do not create "work-arounds"
• Drug Alerts can be numerous: interactions, allergies, duplications, and clinical warnings should be brought to the pharmacist's attention
Filing/dispensing
• Incorrect reading of labels can lead to mix-ups
• Be aware of "confirmation bias" (selecting what is expected or familiar, rather than what is actually written)
• Physically separate look-alike labels and packaging to help reduce errors
• Technology such as bar code scans also may help reduce errors
Point of sale
• Use a second identifier (address or date of birth in addition to name) at point of sale
• Review each medication name with patient or caregiver at point of sale for final check
• Develop procedure for dispensing of high-alert medications
• Establish protocol to refer patient for pharmacist counseling when there is a major change in administration or dosing instructions (or follow state protocol)
Error reporting
• Discuss internal errors among pharmacists, technicians, and clerks
• Refer to ISMP Medication Safety Alert (Community/Ambulatory Care, Acute Care, or Long-term Care Editions) to review errors reported nationally, and prevention strategies9
|
reporting ERRORS
Obviously, no one is eager to come forward and report that he or she has committed or overlooked a mistake related to a medical matter. As noted earlier, errors are rarely made by someone who intends harm, but tend to occur when the person is distracted, overworked, or working within a system or environment that increases the chance of errors. There are two general schools of thought when it comes reporting: one is the “blame game,” in which the person who made the error is punished, perhaps by being fired or having reduced responsibilities. This encourages the staff to cover up errors, which does not address the underlying cause. An alternative approach, sometimes known as “just culture,” accepts that the error is not necessarily the fault of the individual. In this approach, the person is encouraged to come forward, and the staff explores ways to fix flaws in the system to prevent the error from occurring again. Research has shown that the “just culture” approach is a more effective way to reduce and prevent errors while maintaining staff morale.10
Several third–party medication error–reporting services are available to help inform regulators, the FDA, and policymakers about drug–related problems and errors (Table 3).12 The FDA reviews reports coming from some of these sources.
| Table 3. Medication Error Tracking Services |
|
Food and Drug Administration
(800) 332-1088
www.fda.gov/medwatch.htm
Accepts reports from consumers and health professionals about products regulated by the FDA, including drugs and medical devices, through MedWatch, the FDA's safety information and adverse event reporting program.
Institute for Safe Medication Practices
(215) 947-7797
www.ismp.org
Accepts reports from consumers and health professionals related to medication. Publishes Safe Medicine, a consumer newsletter on medication errors.
U.S. Pharmacopeia
(800) 23-ERROR (233-7767)
www.usp.org
The Medication Errors Reporting (MER) Program, in cooperation with the Institute for Safe Medication Practices (ISMP), is a voluntary national medication error reporting program.
MedMARX
www.medmarx.com
This is USP's anonymous medication error reporting program used by hospitals. Data are not submitted to the FDA.
|
SAFE DISPOSAL OF EXPIRED/UNWANTED MEDICATIONS
The process of MTM often reveals the presence of old, expired, or unused medications that the patient still has in his or her possession. Patients often ask pharmacy technicians what to do with expired or unwanted medications. Some pharmacies are able to dispose of medications on the patient’s behalf, but this may depend on local regulations, pharmacy policies, and the type of medication. For example, there are very specific disposal policies associated with controlled substances like opioids. Pharmacy technicians should be aware of safe medication storage and disposal practices, which may include take–back programs for unwanted medications and services that offer prepaid envelopes for mail–in disposal of medications. In preparation for MTM, pharmacy technicians should be aware of the local regulations for medication disposal, as well as any specific institutional recommendations. The Office of Diversion Control of the U.S. Drug Enforcement Administration provides detailed information about safe drug disposal.13 Another resource is available in the article, “How to Dispose of Unused Medication” from the FDA’s website (see reference 14 for link).14 Technicians should be aware that more complicated steps (such as bringing the medications to a disposal site) are unrealistic for many patients.
SUMMARY AND CONCLUSIONS
Safety and error prevention are integral aspects of MTM practice. Pharmacists have extensive training and experience in error prevention and detection, and should look for ways to incorporate these skills into MTM recommendations. Safety considerations must be balanced with the efficacy goals for the therapeutic regimen. Additional resources for the pharmacist and technician are provided in the box, Medication Safety Web Resources.
REFERENCES
- Aspden P, Wolcott J, Bootman JL, et al (Eds). Preventing Medication Errors. Institute of Medicine, Committee on Identifying and Preventing Medication Errors. National Academies Press, 2007.
- Kohn LT, Corrigan JM, Donaldson MS, Ed. To Err Is Human: Building a Safer Health System. Institute of Medicine: Committee on Quality of Health Care in America. Washington, DC: National Academy of Sciences, 2014.
- National Priorities Partnership. Preventing Medication Errors: a $21 billion opportunity. National Quality Forum, Updated February 2011. Available at: https://psnet.ahrq.gov/resources/resource/20529.
- Tariq RA, Vashisht R, Scherbak Y. Medication Errors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Updated June 15, 2020]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK519065/.
- Agency for Healthcare Research and Quality (AHRQ). Reducing and preventing adverse drug events to decrease hospital costs. Research in Action, Issue 1. Available at: http://archive.ahrq.gov/research/findings/factsheets/errors-safety/aderia/ade.html.
- Health Research & Educational Trust (February 2017). Adverse Drug Event (ADE) Change Package: Chicago, IL: Health Research & Educational Trust. Available at: https://www.hqinstitute.org/sites/main/files/file-attachments/p3_hret_ade_change_package.pdf.
- National Coordinating Council for Medication Error Reporting and Prevention. NCC MERP Index for Categorizing Medication Errors. Available at: http://www.nccmerp.org/categorizing-medication-errors-index-color.
- Lisby M, Nielsen LP, Mainz J. Errors in the medication process: frequency, type, and potential clinical consequences. Int J Qual Health Care. 2005;17(1):15-22.
- Institute for Safe Medication Practices. ISMP Safety Alert! Community/Ambulatory Care. Available at: https://http://www.ismp.org/Newsletters/ambulatory/.
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). Reducing medication errors associated with at-risk behaviors by health professionals. Available at: http://www.nccmerp.org/reducing-medication-errors-associated-risk-behaviors-healthcare-professionals.
- Reason J. Human error: models and management. BMJ. 2000;320(7237):768-770.
- U.S. Food & Drug Administration. Strategies to reduce medication errors: working to improve medication safety. Available at: https://www.fda.gov/drugs/drug-information-consumers/working-reduce-medication-errors.
- U.S. Drug Enforcement Administration. Office of Diversion Control. Drug disposal information. Available at: http://www.deadiversion.usdoj.gov/drug_disposal/index.html.
- U.S. Food & Drug Administration. How to dispose of unused medicines. Updated 9/10/2020. Available at: https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.