
Expired activity
Please go to the PowerPak
homepage and select a course.
Emerging Treatment Options for RET-Driven Non-Small Cell Lung and Thyroid Cancers: Preparing Pharmacists to Optimize Patient Care
INTRODUCTION
In many patients with advanced non–small-cell lung cancer (NSCLC) or thyroid cancer, multiple targetable oncogenic mutations can occur that affect receptor tyrosine kinases involved in key cell signaling, proliferation, and survival pathways. Examples include alterations in genes for the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), and REarranged during Transfection (RET) in NSCLC and neurotrophic tyrosine receptor kinase (NTRK), V-raf murine sarcoma viral oncogene homolog B1 (BRAF), and RET in selected thyroid tumors. Multikinase inhibitors (MKIs) targeting multiple receptor tyrosine kinases have demonstrated efficacy and have been approved for the treatment of NSCLC (e.g., gefitinib, erlotinib, afatinib, crizotinib) and thyroid cancer (e.g., cabozantinib, lenvatinib, vandetinib).1,2 Their lack of specificity and associated toxicities, however, have led to the search for and clinical development of next-generation inhibitors with greater selectivity for RET. These agents have shown good efficacy and safety when evaluated specifically for the treatment of patients whose tumors have RET alterations, including those with advanced NSCLC and thyroid cancer.3-6
RET BIOLOGY AND PATHOBIOLOGY
The RET gene encodes a 150-KDa plasma membrane-bound receptor tyrosine kinase that is expressed on various neuroendocrine and neuronal tumors. Signaling through the RET receptor is normally mediated by the glial-derived neurotrophic factor (GDNF) ligand family that includes GDNF, neurturin (NRTN), persephin (PSPN), and artemin (ARTN).7 These ligands bind to a coreceptor, and this complex subsequently interacts with the RET receptor extracellular domain (Figure 1).8 Oncogenic activation of RET can occur due to somatic or germline RET mutations or fusions with other genes, resulting in ligand-independent constitutive activation of the RET kinase. This, in turn, triggers unregulated downstream signaling of RAS and PI3K/AKT/mTOR pathways, with stimulation of cell growth, angiogenesis, and metastasis.9
| Figure 1. Structure of Wild-Type and Oncogenic RET Genes8 |
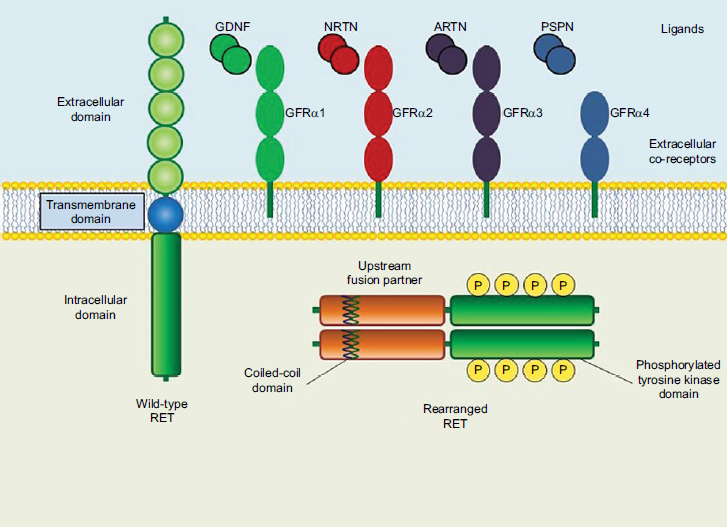 |
| ARTN, artemin; GDNF, glial-derived neurotrophic factor; GFR, GDNF family receptor; NRTN, neurturin; P, phosphorylated; PSPN, persephin; RET, REarranged during Transfection. |
Various RET alterations have been identified in a variety of human solid tumors. RET mutations can occur in the germ line or may be acquired somatically, whereas RET fusions are generally somatic alterations.8 In a recent survey, nearly 57,000 patients with various tumor types were analyzed for RET genetic changes. The overall incidence of RET alterations was 2.4%, which included fusions (6.4%), missense mutations (82.5%), truncating mutations (9.6%), and in-frame mutations (1.5%). RET amplification was detected in 1.5% of all samples. RET gene fusions were seen in 0.15% of samples and were most common in patients with NSCLC (62.6%), thyroid cancer (18.6%), colorectal cancer (5.5%), prostate cancer (4.4%), and gastric cancer (3.3%).10
RET rearrangements are found in 1% to 2% of unselected NSCLC patients.11 A meta-analysis of nearly 7,000 patients with NSCLC indicated that RET fusions are significantly more common in females (especially Asians) and younger patients (<60 years of age), as well as nonsmokers and those with adenocarcinoma12; other data support the observation that RET rearrangements are found more often in never smokers or light smokers.7 In a study of 215 NSCLC patients with RET, ALK, or ROS1 fusions, RET alterations were more common in individuals initially presenting with stage I to II disease and more frequently associated with neuroendocrine histology; RET-positive patients also tended to be older at initial diagnosis.13 In this study, primary tumors were primarily solid, with no associated air bronchograms, cavitation, or calcification (Figure 2).13
| Figure 2. Representative Imaging of a Patient with RET-Rearranged NSCLC13 |
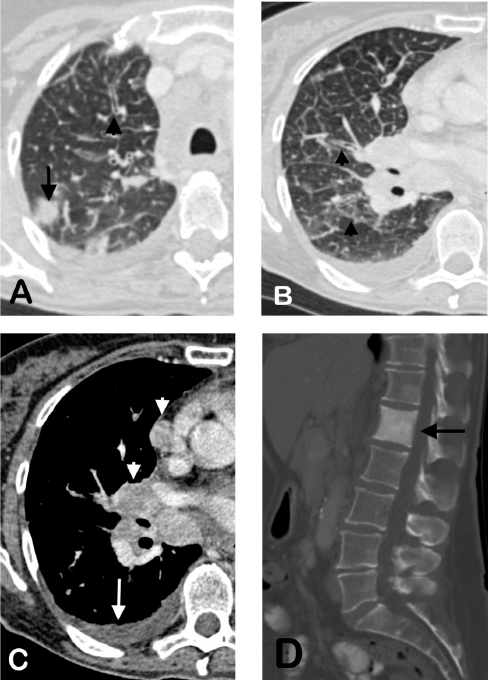 |
Pretreatment computed tomography scans of a 62-year-old female with RET mutation-positive NSCLC. A solid nodule can be seen in the peripheral right upper lobe (A, arrow), along with associated septal with peribronchial thickening consistent with lymphangitic carcinomatosis (A, B, arrowheads). Associated malignant pleural effusion (C, arrow), mediastinal and hilar lymphadenopathy (C, arrowheads), and sclerotic osseous metastasis of the first lumbar vertebral body (D, arrow) are also evident.
NSCLC, non–small-cell lung cancer; RET, REarranged during Transfection. |
RET rearrangements are typically mutually exclusive of other oncogenic driver mutations in lung cancer such as EGFR, ALK, and ROS1. Results of a small study suggested that, in NSCLC patients with RET rearrangement, the presence of a concurrent TP53 mutation is associated with poorer survival.14 Other markers such as tumor PD-L1 expression and tumor mutation burden level were not prognostic for survival in this patient population.
The first RET fusion partner identified in NSCLC tumors was with the kinesin family 5B (KIF5B) gene.7 At least 13 different RET fusion partners have now been identified in NSCLC, including CCDC6 (coiled-coil domain-containing protein 6), TRIM33 (tripartite motif-containing 33), NCOA4 (nuclear receptor coactivator 4), MYO5C (myosin VC gene), EPHA5 (EPH receptor A5 gene), ERC1 (ELKS/RAB6-interacting/CAST family member 1), FRMD4A (FERM domain-containing 4A), CLIP1 (CAP-Gly domain-containing linker protein family member 1), PICALM (phosphatidylinositol-binding clathrin assembly protein), TRIM24 (tripartite motif-containing 24), and RUFY2 (RUN and RYVE domain-containing 2).8 These fusions all result in ligand-independent RET receptor dimerization and activation.
Activating RET point mutations are frequently seen in medullary thyroid cancer (MTC) and are detected in approximately 50% of sporadic cases; RET gene rearrangements are also possible.15 Virtually all familial MTC cases have germline activating RET mutations, and these can also occur in the related multiple endocrine neoplasia type 2 (MEN2A and MEN2B) syndromes.16 RET rearrangements resulting in fusion proteins are seen in 20% to 40% of patients with papillary thyroid cancer (PTC), and in Asian populations, these are more common in younger patients (≤45 years old).17
Screening for RET alterations
Genomic screening of patients with NSCLC and thyroid cancer is important for identification and characterization of oncogenic driver mutations since these can guide selection of therapy. In NSCLC, this includes testing for genetic alterations in RET, EGFR, ALK, ROS1, BRAF, and MET exon 14.1 Similarly, in thyroid cancer, genomic testing is indicated to identify targetable mutations, including RET and NTRK gene fusions, and to identify patients with familial RET mutations.2 Molecular screening may utilize single-gene tests to detect the presence of individual mutations or involve multigene assays.
Detection of sensitizing mutations in such tumors can guide rational selection of targeted agents specific for these alterations, such as use of RET tyrosine kinase inhibitors (TKIs) for NSCLC patients with RET fusions or erlotinib for NSCLC patients with EGFR alterations. Patients with progressive, metastatic MTC bearing RET M918T or RAS mutations may have a greater response to cabozantinib.18 A poor response to vandetinib was seen in NSCLC patients with a KIF5B-RET fusion compared to those with with CCDC6-RET rearrangements.19 In papillary carcinoma, use of larotrectinib is indicated in patients with NTRK gene fusion-positive tumors, and dabrafenib/trametinib can be used for those with BRAF V600E mutations.2 In select cases, genomic information might also guide surgical treatment. In MTC, the presence of a RET p.Val804Met mutation was associated with a very low (4%) penetrance, suggesting that prophylactic thyroidectomy may not be necessary for such patients.20
Several methods exist for detection and characterization of RET alterations in tumors. Reverse transcription polymerase chain reaction (RT-PCR) has been widely used to detect known (but not unknown) RET fusions, while fluorescence in situ hybridization (FISH) can detect both but involves considerable expertise and cost. A combination RT-PCR and FISH has been proposed as a practical means of detecting RET fusions in thyroid cancer.21 Next-generation sequencing (NGS) has become widely used since it can identify point mutations, fusions, amplifications, and other alterations in multiple target genes, including those affecting RET.22 Cell-free circulating tumor DNA (cfDNA) has been used to detect somatic activating alterations in RET and other oncogenes.23 This is a noninvasive method of genomic profiling that could also be useful for monitoring response to therapy. However, use of cfDNA in NSCLC is often limited to cases in which the patient is unable to undergo a more invasive tumor biopsy or when there is insufficient material for molecular analysis following initial pathologic confirmation of the disease.
Testing guidelines developed by the National Comprehensive Cancer Network (NCCN) recommend that all eligible patients with metastatic NSCLC should undergo molecular profiling to detect RET fusions or mutations affecting EGFR, ALK, ROS1, BRAF, or MET exon 14 skipping mutations.1 Similarly, genomic testing for targetable alterations in advanced thyroid cancer can guide treatment decisions for patients with specific RET mutations or genetic alterations affecting BRAF, ALK, NTRK, and mTOR.2 Additionally, for individuals diagnosed with inherited MTC, familial testing is indicated to identify potential carriers of RET mutations.
At present, no United States Food and Drug Administration (FDA)-approved test exists for detecting RET fusions or mutations, so such screening is best accomplished through laboratory testing.
KEY CLINICAL TRIAL DATA FOR RET REARRANGEMENT-POSITIVE NSCLC AND THYROID CANCER
NSCLC
Multikinase inhibitors
MKIs have shown varying degrees of efficacy in patients with RET-rearranged advanced NSCLC (Table 1). For example, vandetinib monotherapy resulted in an overall response rate (ORR) of 53% in one study in previously treated patients,24,25 while in another, the combination of vandetinib and everolimus, produced a comparable outcome.26 Other MKIs such as lenvatinib and cabozantinib have shown less activity.27-29
| Table 1. Pivotal Trials of RET Inhibitors in Advanced NSCLC |
| Study |
Trial |
Patient population |
N |
Regimen |
Objective response ratea |
Median progression-free survival, months |
Median duration of response, months |
| Yoh et al 201724, 25 |
LURET phase II |
Previously treated RET-rearranged advanced NSCLC |
19 |
Vandetanib |
53% (95% CI, 31–74) |
6.5 (95% CI, 2.8–8.5) |
NR |
| Lee et al 201719 |
Phase II |
Metastatic or recurrent RET-rearranged NSCLC, progressive disease following platinum-based doublet chemotherapy |
18 |
Vandetanib |
18% |
4.5b |
NR |
| Velcheti et al 201627,28 |
Phase II |
RET fusion-positive lung adenocarcinoma |
25 |
Lenvatinib |
16% (95% CI, 4.5–36.1) |
7.3 (95% CI, 3.6–10.2) |
NR |
| Drilon et al 201629 |
Phase II |
Metastatic or unresectable RET-rearranged NSCLC |
26 |
Cabozantinib |
28% (95% CI, 12–49) |
5.5 (95% CI, 3.8–8.4) |
7.0 (95% CI, 3.7–38.9) |
| Subbiah et al 201826 |
Phase I |
Advanced RET-rearranged NSCLC |
13 |
Vandetanib + everolimus |
54%c |
4.4 (95% CI, 3.4 to NR)c |
NR |
| Drilon et al 20203 |
LIBRETTO-001 phase I/II |
RET fusion-positive NSCLC |
144
Systemic treatment-naïve (n=39) Prior systemic therapy (n=105) |
Selpercatinib |
90% (95% CI, 76–97) 70% (95% CI, 60–78) |
NE (95% CI, 13.8 to NE) 18.4 (95% CI, 16.4–24.8) |
NE (95% CI, 12.0 to NE) 20.3 (95% CI, 15.6–24.0) |
| Gainor et al 202030 |
ARROW phase I/II |
RET fusion-positive NSCLC
(median of 2 prior therapies; 39% with brain metastases) |
116 Systemic treatment-naïve (n=26) Prior systemic therapy (n=80) |
Pralsetinib |
65% (95% CI, 55–73)d |
NR |
NRe |
aInvestigator assessment.
b95% CI not reported.
cIn patients with tumors determined to be RET fusion-positive as detected by next-generation sequencing, a 70% objective response rate was observed with a median progression-free survival of 8.0 months (95% CI, 0.1–1.1).
dObjective response rate of 61% (95% CI, 50–72) in patients previously treated with platinum-based chemotherapy.
eResponse duration of ≥6 months achieved in 6 of 32 responders.
CI, confidence interval; NE, not estimable; NR, not reported; NSCLC, non–small-cell lung cancer; RET, REarranged during Transfection. |
A retrospective analysis of data from the international Global Multicenter RET Registry (GLORY) evaluated trials in which a total of 165 patients with RET-rearranged NSCLC were treated with various MKIs (first- to eighth-line therapy). Of the 9 drugs evaluated, only cabozantinib, sunitinib, and vandetanib showed any substantial efficacy, with response rates ranging from approximately 18% to 37%. For the entire group, the median progression-free survival (PFS) was 2.3 months (95% confidence interval [CI], 1.6–5.0) and median overall survival was 6.8 months (95% CI, 3.9–14.3).31 The authors concluded that available MKIs have limited activity in patients with RET-rearranged NSCLC.
Selpercatinib
Recent trials have assessed more specific RET inhibitors in this patient population. The LIBRETTO-001 phase I/II basket trial evaluated the RET inhibitor selpercatinib in both treatment-naïve and heavily pretreated patients for a variety of RET-driven solid tumors. In a cohort of 39 systemic treatment-naïve patients with RET fusion-positive NSCLC, the investigator-assessed ORR was 90% (95% CI, 76–97) and median PFS was not estimable (95% CI, 13.8 to not estimable). In the cohort of 105 patients with prior systemic therapy, the ORR was 70% (95% CI, 60–78) and median PFS was 18.4 months (95% CI, 16.4–24.8). Of note, intracranial responses were seen in 10 of 11 patients with measurable brain metastases, with a median central nervous system (CNS) duration of response exceeding 10 months.3
Pralsetinib
Pralsetinib is a selective RET inhibitor active against oncogenic RET alterations, including the most common RET fusions and those that confer resistance to MKIs.4 In the phase I/II ARROW study, patients with RET fusion-positive advanced NSCLC received pralsetinib daily. Preliminary data have been reported for 79 patients who had received a median of 2 prior therapies (39% had brain metastases). The ORR was 65% among all response-evaluable patients and 61% in those who had received prior platinum-based chemotherapy. Responses were seen across various RET fusion genotypes. Intracranial activity was observed in some patients, with shrinkage of brain metastases.30
RXDX-105
RXDX-105 is a vascular endothelial growth factor receptor (VEGFR)-sparing MKI with RET inhibitory activity. This agent demonstrated responses in patients with RET fusion-positive NSCLC (ORR, 19%) but only in those without the most common RET fusion (KIF5B–RET).32 Clinical development of RXDX-105 for this indication has been halted.
Thyroid cancer
Multikinase inhibitors
Previous studies have evaluated MKIs for advanced thyroid cancer and have reported mixed results (Table 2).5,6,33-37 A phase III trial of vandetanib in patients with metastatic MTC reported an ORR of 45%,33 while another trial of cabozantinib in this patient population achieved an ORR of 28%.35 In patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer, treatment with lenvatinib produced an ORR of 65%.36 These trials were not limited to patients with RET alterations, however.
| Table 2. Pivotal Trials of RET Inhibitors in Advanced Thyroid Cancer |
| Study |
Trial |
Patient population |
N |
Regimen |
Objective response ratea |
Median progression-free survival, months |
|
Wirth et al 20205
|
LIBRETTO-001 phase I/II
|
RET-mutated MTC with prior cabozantinib and/or vandetanib therapy
RET fusion-positive thyroid cancers with prior cabozantinib and/or vandetanib therapy
|
55
19
|
Selpercatinib
Selpercatinib
|
62% (95% CI, 48–75)
58% (95% CI, 34–80)
|
27.4 (95% CI, 13.7 to NE)
NE (95% CI, 10.0 to NE)
|
|
Taylor et al 20196
|
ARROW phase I/II
|
RET-mutated MTC (58% with prior multikinase inhibitor therapy)
|
60
|
Pralsetinib
|
47%
|
NR
|
|
Schöffski et al 201235
|
EXAM phase III
|
Metastatic MTC
|
330
|
Cabozantinib vs. placebo
|
28% vs. 0%
|
11.2 vs. 4.0; HR 0.28 (95% CI, 0.19–4.0); P<0.0001
|
|
Wells et al 201233,34
|
ZETA phase III
|
Metastatic MTC
|
331
|
Vandetanib vs. placebo
|
45% vs. 13%
|
30.5 vs. 19.3; HR 0.46 (95% CI, 0.31–0.69); P<0.001
|
|
Brose et al 201437
|
DECISION phase III
|
RAI-refractory DTC with disease progression
|
417
|
Sorafenib vs. placebo
|
12% vs. <1%
|
10.8 vs. 5.8; HR 0.59 (95% CI, 0.45–0.76); P<0.0001
|
|
Nair et al 201536
|
303 phase III
|
RAI-refractory DTC with disease progression
|
92
|
Lenvatinib vs. placebo
|
65% vs. 2%
|
18.3 vs. 3.6; HR 0.21 (95% CI, 0.16–0.28); P<0.001
|
aInvestigator assessment.
CI, confidence interval; DTC, differentiated thyroid cancer; HR, hazard ratio; MTC, medullary thyroid cancer; NE, not estimable; NR, not reported; RAI, radioactive iodine; RET, REarranged during Transfection. |
More recent studies evaluated newer RET inhibitors for RET-altered thyroid cancer. In a primary analysis set of 55 patients with RET-mutant MTC enrolled in the selpercatinib LIBRETTO-001 trial who previously received cabozantinib, vandetanib, or both, the ORR (per investigator assessment) was 62% (95% CI, 48–75). Three of these responses occurred in patients with a RET V804L/M gatekeeper mutation associated with resistance to TKIs. With a median follow-up of 14.8 months, the duration of response has not yet been reached (95% CI, 18.4 to not estimable). The median PFS was 27.4 months (95% CI, 13.7 to not estimable). Biochemical (calcitonin and carcinoembryonic antigen) responses occurred in the majority of cases. In a separate cohort of 19 patients with previously treated RET fusion-positive thyroid cancer, an ORR of 58% (95% CI, 34–80) was reported.5
Pralsetinib
In addition to NSCLC, the ARROW study also evaluated pralsetinib in patients with RET-altered MTC and PTC. Preliminary results for 60 patients with RET-mutated MTC, 58% of whom had received prior TKI therapy, were reported. Among response-evaluable patients, the ORR was 47%, with responses noted regardless of prior TKI therapy or RET genotype. At the time of the report, 96% of patients remained on treatment, 15 of whom had a response duration of at least 6 months. Responses were also seen in 2 of 4 patients with PTC.6
NCCN RECOMMENDATIONS FOR RET REARRANGEMENT-POSITIVE NSCLC AND THYROID CANCER
In light of the unique biology of RET-driven NSCLC and thyroid cancer, treatment guidelines were recently revised to support the preferential use of RET inhibitors in selected patients. Current NCCN treatment guidelines indicate that selpercatinib is preferred as initial therapy for those with advanced NSCLC in whom a RET rearrangement is detected prior to initiation of first-line systemic therapy (Table 3).1 Cabozantanib, vandetanib, and selected cytotoxic chemotherapy or immune checkpoint inhibitors may also be considered in certain circumstances. In cases where RET alterations are not discovered until after initiation of first-line systemic therapy, clinicians have the option of completing therapy as planned (including any maintenance therapy) or interrupting such treatment and then initiating selpercatinib, cabozantinib, or vandetanib. Following disease progression on these TKIs, subsequent therapy options include selected chemotherapeutics (e.g., docetaxel, pemetrexed, gemcitabine), bevacizumab, and immune checkpoint inhibitors. Conversely, patients who had not received RET inhibitors in the first-line setting could receive these agents upon progression.
| Table 3. NCCN Treatment Guidelines for RET-Rearranged Advanced NSCLC and Thyroid Cancer |
| NSCLC1 |
Setting |
First-line therapy |
Subsequent therapyb |
|
Advanced or metastatic NSCLC
|
RET rearrangement detected prior to first-line systemic therapy
RET rearrangement detected during first-line systemic therapy
|
Preferred
Useful in certain circumstances
- Complete first-line systemic therapy or interrupt, then initiate selpercatinib, cabozantinib, or vandetaniba
|
Preferred
Useful in certain circumstances
- Other non-RET inhibitor systemic therapy (e.g., platinum, paclitaxel, pemetrexed, immune checkpoint inhibitors)
|
| Thyroid cancer2 |
Setting |
Therapy |
|
Papillary carcinoma
Follicular carcinoma
|
Locally recurrent, advanced, or metastatic RET fusion-positive disease not amenable to RAI therapy
|
|
|
Medullary carcinoma
|
Locoregional, recurrent/persistent, or metastatic RET mutation-positive disease
|
Asymptomatic disease
- Selpercatinib (preferred)
|
Symptomatic disease
|
aCategory 2B: based on lower-level evidence, there is NCCN consensus that the intervention is appropriate.
bFollowing first-line non-RET inhibitor systemic therapy.
NCCN, National Comprehensive Cancer Network; NSCLC, non–small-cell lung cancer; RAI, radioactive iodine; RET, REarranged during Transfection. |
NCCN recommendations regarding treatment of RET-altered locally recurrent, advanced, or metastatic papillary or follicular carcinoma not amenable to RAI therapy have also been recently revised (Table 3).2 Selpercatinib is recommended for RET fusion-positive papillary and follicular carcinoma, and it can also be considered for treatment of bone and CNS metastases in these patients. For recurrent or persistent/metastatic medullary carcinoma, selpercatinib is recommended for asymptomatic or symptomatic patients with RET mutation-positive disease. This agent can also be considered for treatment of unresectable disease that is symptomatic or progressing based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
BRAIN METASTASES
Among patients with advanced NSCLC, up to 64% will develop brain metastases during the course of their disease.38 In contrast, brain metastases are very rarely observed in most thyroid cancers. Prognosis is poor for NSCLC patients with brain metastases, with a median overall survival of 7 months reported in one study.39 Some data suggest that RET-rearranged NSCLC is associated with a substantial cumulative incidence of brain metastasis. A global registry study of patients with stage IV RET-rearranged NSCLC found a 25% incidence of brain metastasis at the time of diagnosis of metastatic disease.40 Although data are limited, responses to MKIs in this patient population were poor, with a median PFS (intracranial and extracranial) of 3.9 months (95% CI, 2.0–4.9). In a retrospective analysis, 32% of all patients with RET fusion-positive NSCLC had brain metastases at initial diagnosis.13 The incidence of brain metastases in this subgroup was comparable to that seen in patients with ALK fusions (25%) but greater than those with ROS1-mutated NSCLC (10%). The estimated cumulative (lifetime) incidence of brain metastases was also significantly different for patient subgroups (ALK > RET > ROS1). Another retrospective analysis of data from 552 patients with advanced NSCLC found that RET gene fusions (P=0.003), EGFR mutations (P=0.012), and ALK gene fusions (P=0.015) were independent risk factors for brain metastasis, in addition to other variables.41 Together, these data indicate that brain metastasis is relatively common in patients with RET fusion-positive NSCLC and is associated with a worse prognosis and poor response to MKIs.
RESISTANCE TO RET INHIBITORS
Both primary and secondary resistance to MKIs have been documented in NSCLC, arising through secondary mutations or amplification affecting the target gene or alterations in bypass signaling mechanisms.42 In the case of RET-altered disease, primary resistance is suggested by the fact that not all patients respond to RET inhibitors. Secondary resistance to these agents is likely to develop with prolonged treatment, as seen with MKIs. Resistance to MKIs often develops due to secondary V804L/M gatekeeper mutations affecting the RET kinase domain. Additional mechanisms for resistance to RET inhibitors, observed in vitro or in vivo, include missense RET mutations, amplification of MDM2 and YAP, and mutations affecting the EGFR and MAPK pathways.8,43 To date, 14 RET kinase domain mutations have been identified that confer in vitro resistance to vandetanib, lenvatinib, cabozantinib, and/or nintedanib, depending on which receptor domain is affected by the mutation; some of these mutations have also been found in patients treated with RET inhibitors.44
Currently, clinical data on secondary resistance to RET inhibitors are limited, but this may increase with expanded use of such therapy. Mutation analysis to identify patient-specific bypass resistance mechanisms could suggest appropriate, effective treatments (e.g., use of MDM2 blockers plus RET inhibitors in patients with MDM2 amplification, or other combination regimens) and help guide sequencing of therapies. Biomarker studies may also aid in early identification of resistance and thus guide clinical decision-making when resistance does develop. In MTC, for example, one study found that increases in serum calcitonin levels (>40%) could signal tumor progression and may be an early indicator of TKI resistance.45
NOVEL RET INHIBITORS
Other new RET inhibitors are in early-stage development, with some now in phase I trials. Many current inhibitors affect mutations that lie outside the RET kinase active site; however, these drugs are much less effective against mutations involving the active site (e.g., V804L/M). In contrast, select investigational agents show activity against RET mutations within the active site.46 Some of these compounds inhibit RETV804M kinase but not the wild-type enzyme and thus could be useful for treatment of resistance to current RET inhibitors. Examples include TPX-0046 (a RET/SRC inhibitor)47; GSK3179106 (a selective RET inhibitor)48; BOS172738 (a RET/VEGFR2 inhibitor)49; and salinomycin and its derivatives.50 Other MKIs that have shown inhibition of RET in preclinical models include dovitinib, alectinib, motesanib, danusertib, and apatinib.16
PHARMACIST IMPLICATIONS IN OPTIMIZING PATIENT OUTCOMES
Adherence
Since RET inhibitors are orally administered, patient adherence is critical for optimizing outcomes. Poor adherence can lead to decreased efficacy, increased healthcare resource utilization, and treatment failures. Long-term therapy requires strict adherence to dosage regimens, so continued monitoring and management of patients over the course of their disease is essential.
Suboptimal adherence and early discontinuation of therapy can result from poor management of treatment-associated toxicity. A small study of patients with refractory metastatic thyroid cancer who received up to 4 cycles of MKIs (sunitinib, sorafenib, or vandetanib) found that 21% discontinued therapy due to treatment-related adverse effects.51 Consistent monitoring by pharmacists can help patients receiving RET inhibitors remain on treatment as prescribed and thus enhance outcomes. Additionally, pharmacists can counsel patients to identify factors that make adherence challenging (e.g., side effects, cost/insurance coverage, outcome expectations) and work with patients to overcome these obstacles. Pharmacist-directed intervention strategies, coupled with consistent monitoring and patient education, can help patients remain on RET inhibitors as prescribed.52
Adverse events
Pharmacists need to be well informed about treatment-related toxicities related to RET inhibitors in order to minimize adverse effects and ensure prompt intervention and treatment. Toxicities associated with MKIs are generally low grade, although, as reported in some studies, their incidence can be significant (e.g., 77% diarrhea and 63% fatigue with pazopanib for advanced MTC, and 56% diarrhea with vandetanib).53 In clinical trials of RET inhibitors for NSCLC and thyroid cancer, toxicities were of lower grade and usually did not require treatment discontinuation. Familiarity with the most common treatment-emergent adverse events with RET inhibitors will help pharmacists reduce the risk of these toxicities, facilitate prophylaxis, and ensure prompt, effective treatment.
In the LIBRETTO-001 trial of selpercatinib in RET-altered thyroid cancer, adverse events were mostly low grade. The most common (≥15%) treatment-related adverse events (TRAEs) included xerostomia, diarrhea, hypertension, and increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT); these were mostly grade 1 or 2 and resulted in treatment discontinuation in 1.7% of patients.5 According to selpercatinib prescribing information, patients should be monitored for hypertension and aminotransferase levels prior to and during treatment, and at-risk patients should be monitored for possible QT interval prolongation.54 Selpercatinib should be dose-reduced or discontinued following certain grade 3 or 4 adverse events including hepatotoxicity, hypertension, QT interval prolongation, hemorrhagic events, hypersensitivity reactions, and other toxicities.
In the ARROW trial of pralsetinib in RET fusion-positive NSCLC, TRAEs were generally low grade and included increased AST (22%), hypertension (18%), increased ALT (17%), constipation (17%), fatigue (15%), and decreased neutrophils (15%).30 In the thyroid cancer cohort, TRAEs included decreased white blood cells (23%), increased AST (17%), increased ALT, blood creatinine, and phosphate, hypertension, and decreased neutrophils (all 15%). The incidence of grade ≥3 TRAEs in both cohorts was 28%, and no events required discontinuation.
Familiarity with the adverse event profiles associated with RET inhibitors will help pharmacists be better aware of TRAEs and aid in both adverse event prophylaxis and management.
Patient education
Pharmacists can play a pivotal role in educating cancer patients about RET-targeted therapy and potential adverse effects, including early identification and reporting of treatment-related toxicities.
Pharmacist-directed patient education and support can help patients and clinicians better manage toxicities, maintain adherence, and minimize treatment interruptions or discontinuations. For example, a pharmacist medication therapy management program for elderly cancer patients (including some with NSCLC) was shown to be effective in managing drug-related problems and significantly improved patients’ satisfaction levels.55 Similarly, a wellness education intervention in NSCLC patients undergoing treatment with the EGFR MKI icotinib was found to improve their quality of life.56
Pharmacists are well positioned to answer patient questions about dosing, adherence, and potential drug–drug or drug–food interactions that could limit absorption and bioavailability of RET inhibitors. Older patients and those with comorbidities, in particular, are likely to be at greater risk for drug–drug interactions due to polypharmacy and could, therefore, benefit from such support. Patient education can thus help reduce toxicity and enhance quality of life. This will also allow patients to participate in shared treatment decision-making, which has proven to be effective in enhancing patient engagement and improving outcomes.57
CONSIDERATIONS FOR FORMULARY DECISION-MAKING AND PRIOR AUTHORIZATION MANAGEMENT
Approximately 2% of NSCLC cases, 10% to 20% of PTC, and 60% to 90% of MTC will have a RET fusion or mutation, which must be documented before initiation of selpercatinib therapy (see Screening for RET Alterations above).
Currently, selpercatinib is the only RET inhibitor approved by the FDA for patients with RET fusion-positive NSCLC and for those with advanced or metastatic RET mutant/fusion-positive thyroid cancer. (Approval of pralsetinib for RET fusion-positive NSCLC is expected by the end of 2020.58)
Selpercatinib is marketed as 40- and 80-mg capsules. The recommended dose is 120 mg twice daily for patients weighing less than 50 kg, and 160 mg twice daily for those 50 kg or more. The wholesale acquisition cost for a 30-day supply of selpercatinib (160 mg by mouth twice daily) was set at $20,600.59
CONCLUSION
RET has been demonstrated to be a rational, druggable target in NSCLC and thyroid cancer patients with RET alterations. The clinical development of RET inhibitors–and approval to date of one such agent–has confirmed their efficacy in eligible patients. Such therapy has the potential to increase long-term survival and improve quality of life, with less toxicity than other therapies.
Numerous clinical trials of RET inhibitors in RET-altered advanced NSCLC and thyroid cancer are ongoing or planned (Table 4).60 In RET fusion-positive advanced NSCLC, 2 phase III trials are separately comparing either pralsetinib or selpercatinib to standard platinum-based chemotherapy (with or without pembrolizumab). Another phase III trial is evaluating selpercatinib versus cabozantinib or vandetinib as first-line therapy in patients with RET-mutant MTC.
| Table 4. Selected Ongoing Clinical Trials of RET Inhibitors in Advanced NSCLC and Thyroid Cancer60 |
| NCT trial number (acronym) |
Agent/regimen |
Phase |
Patient population |
N |
Primary outcome measure |
Estimated primary completion date |
|
| NSCLC |
| NCT03037385 (ARROW) |
Pralsetinib |
I/II |
Advanced NSCLC with RET fusion |
527a |
ORR |
December 2021 |
NCT04222972
(AcceleRET) |
Pralsetinib vs. platinum-based chemotherapy (± pembrolizumab) |
III |
Metastatic NSCLC with RET fusion (first-line setting) |
250 |
PFS |
September 2023 |
NCT04194944
(LIBRETTO-431) |
Selpercatinib vs. pemetrexed + platinum-based chemotherapy (± pembrolizumab) |
III |
Advanced NSCLC with RET fusion |
400 |
PFS |
December 2023 |
| NCT04204928 |
Pralsetinib |
Preapproval access program |
Nonresectable or metastatic NSCLC with RET fusion or RET-mutated advanced MTC |
NA |
NA |
NA |
| Thyroid cancer |
| NCT03690388 (COSMIC-311) |
Cabozantinib |
III |
RAI-refractory DTC resistant to VEGFR-targeted therapy |
300 |
ORR, PFS |
July 2020 |
| NCT03037385 (ARROW) |
Pralsetinib |
I/II |
Advanced MTC |
527a |
ORR |
December 2021 |
NCT03157128
(LIBRETTO-001) |
Selpercatinib |
I/II |
Advanced solid tumors including RET fusion-positive solid tumors, MTC, and other tumors with RET activation |
970 |
ORR |
March 2022 |
NCT04211337
(LIBRETTO-531) |
Selpercatinib vs. cabozantinib or vandetanib |
III |
Progressive, advanced, TKI-naïve RET-mutant MTC |
400 |
TFFS |
February 2023 |
a“Basket” trial evaluating multiple tumor types including NSCLC and thyroid cancer.
DTC, differentiated thyroid cancer; MTC, medullary thyroid cancer; NA, not applicable; NSCLC, non–small-cell lung cancer; ORR, overall response rate; PFS, progression-free survival; RAI, radioactive iodine; RET, REarranged during Transfection; TFFS, treatment failure-free survival; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. |
Pharmacists and other healthcare professionals should recognize that all patients with advanced NSCLC or thyroid cancer must undergo genomic screening as early as possible to guide selection of the optimal targeted therapy, including the use of RET inhibitors for patients with RET alterations. Continued research may help identify biomarkers that can better inform patient selection and predict outcomes with RET inhibitor therapy. There may also be opportunities for these agents to be used as adjuvant therapy for advanced NSCLC, although, currently, no such clinical trials are ongoing. Future studies may explore the potential of RET inhibitors in combination with other targeted agents or immunotherapy to further enhance response and obviate the development of resistance.
|
Updates: June 25, 2021
- The FDA granted Breakthrough Therapy designation to cabozantinib as a potential treatment for patients with differentiated thyroid cancer that has progressed following prior therapy and who are radioactive iodine–refractory (if radioactive iodine is appropriate). In December 2020, at a planned interim analysis, the phase III COSMIC-311 pivotal trial met its co-primary endpoint, demonstrating a significant reduction in the risk of disease progression or death of 78% with cabozantinib vs placebo (hazard ratio = 0.22, 96% confidence interval = 0.13 – 0.36, P < .0001) in patients with radioactive iodine–refractory differentiated thyroid cancer who had disease progression after up to two prior VEGFR-targeted therapies. The safety profile was consistent with that previously observed for cabozantinib.
Reference:
Brose MS, Robinson B, Sherman SI, et al. Cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer who have progressed after prior VEGF-targeted therapy: results from the phase 3 COSMIC-311 trial. J Clin Oncol. 2021;39:(suppl 15; abstr 6001). doi:10.1200/JCO.2021.39.15_suppl.6001
Updates: March 23, 2021
- The NCCN guidelines for thyroid cancer have been updated to include pralsetinib. Pralsetinib is
listed in the NCCN guidelines as a category 2A option for patients with RET-fusion positive
papillary carcinoma and follicular carcinoma. Pralsetinib is listed in the NCCN guidelines as a
preferred regimen for patients with RET-mutation positive medullary carcinoma and anaplastic
carcinoma.
Reference:
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®). Thyroid Carcinoma. Version 3.2020. Published February 2, 2021.
Subbiah et al. Clinical activity of the RET inhibitor pralsetinib in patients with RET fusion+ solid
tumors. Presented at the American Society of Clinical Oncology Annual Meeting; May 29-31, 2020.
Updates: December 11, 2020
-
The National Comprehensive Cancer Network (NCCN) guidelines (Version 1.2021) recommend molecular testing of NTRK1/2/3 in patients diagnosed with advance or metastatic non-small cell lung cancer.
Reference:
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 1.2021. Published November 25, 2020.
- On September 4, 2020, the Food and Drug Administration granted accelerated approval to pralsetinib for adult patients with metastatic RET fusion-positive non-small cell lung cancer (NSCLC) as detected by an FDA approved test. Efficacy of pralsetinib for RET fusion-positive NSCLC was evaluated in the ARROW trial that enrolled 87 patients previously treated with platinum chemotherapy. The overall response rate (ORR) was 57% (95% CI: 46%, 68%); 80% of responding patients had responses lasting 6 months or longer. Efficacy was also evaluated in 27 patients who never received systemic treatment. The ORR for these patients was 70% (95% CI: 50%, 86%); 58% of responding patients had responses lasting 6 months or longer. Pralsetinib is listed in the NCCN guidelines as a preferred option for the first-line management of advanced or metastatic NSCLC patients with a RET rearrangement detected prior to first-line systemic therapy. Pralsetinib is also listed a preferred subsequent therapy option in this setting.
References:
Gavreto [package insert]. Blueprint Medicines Corporation; Cambridge, MA: 2020.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 1.2021. Published November 25, 2020.
- On December 1, 2020, the Food and Drug Administration approved pralsetinib for adult and pediatric patients 12 years of age and older with advanced or metastatic RET-mutant medullary thyroid cancer (MTC) who require systemic therapy or RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate).
|
REFERENCES
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 6.2020. Published June 24, 2020.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Thyroid Carcinoma. Version 1.2020. Published June 12, 2020.
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383(9):813–24.
- Subbiah V, Taylor M, Lin J. Abstract CT043: Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. Cancer Res. 2018;78(13 suppl):CT043.
- Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825–35.
- Taylor MH, Gainor JF, Hu MI-N, et al. Activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients with advanced RET-altered thyroid cancers. J Clin Oncol. 2019;37(15_suppl):6018.
- Ackermann CJ, Stock G, Tay R, et al. Targeted therapy for RET-rearranged non-small cell lung cancer: clinical development and future directions. Onco Targets Ther. 2019;12:7857–64.
- Bronte G, Ulivi P, Verlicchi A, et al. Targeting RET-rearranged non-small-cell lung cancer: future prospects. Lung Cancer (Auckl). 2019;10:27–36.
- Mendoza L. Clinical development of RET inhibitors in RET-rearranged non-small cell lung cancer: update. Oncol Rev. 2018;12(2):352.
- Andreev-Drakhlin A, Roszik J, Subbiah V. The landscape of RET alterations from 56,970 adult patients with cancer: clinical implications. J Clin Oncol. 2019;37(15_suppl):3106.
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50.
- Lin C, Wang S, Xie W, et al. The RET fusion gene and its correlation with demographic and clinicopathological features of non-small cell lung cancer: a meta-analysis. Cancer Biol Ther. 2015;16(7):1019–28.
- Digumarthy SR, Mendoza DP, Lin JJ, et al. Imaging features and patterns of metastasis in non-small cell lung cancer with RETCancers (Basel). 2020;12(3):693.
- Lu C, Dong X-R, Zhao J, et al. Association of genetic and immuno-characteristics with clinical outcomes in patients with RET-rearranged non-small cell lung cancer: a retrospective multicenter study. J Hematol Oncol. 2020;13(1):37.
- Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–49.
- De Falco V, Carlomagno F, Li H-Y, Santoro M. The molecular basis for RET tyrosine-kinase inhibitors in thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31(3):307–18.
- Ming J, Liu Z, Zeng W, et al. Association between BRAF and RAS mutations, and RET rearrangements and the clinical features of papillary thyroid cancer. Int J Clin Exp Pathol. 2015;8(11):15155–62.
- Sherman SI, Clary DO, Elisei R, et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016;122(24):3856–64.
- Lee S-H, Lee J-K, Ahn M-J, et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol. 2017;28(2):292–7.
- Loveday C, Josephs K, Chubb D, et al. p.Val804Met, the most frequent pathogenic mutation in RET, confers a very low lifetime risk of medullary thyroid cancer. J Clin Endocrinol Metab. 2018;103(11):4275–82.
- Musholt TJ, Staubitz JI, Antonio Cámara RJ, et al. Detection of RET rearrangements in papillary thyroid carcinoma using RT-PCR and FISH techniques — a molecular and clinical analysis. Eur J Surg Oncol. 2019;45(6):1018–24.
- Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23(8):1988–97.
- Rich TA, Reckamp KL, Chae YK, et al. Analysis of cell-free DNA from 32,989 advanced cancers reveals novel co-occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin Cancer Res. 2019;25(19):5832–42.
- Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5(1):42–50.
- Yoh K, Seto T, Satouchi M, et al. LURET: final survival results of the phase II trial of vandetanib in patients with advanced RET-rearranged non-small cell lung cancer. Ann Oncol. 2018;29(suppl_8):viii493–viii547.
- Subbiah V, Cascone T, Hess KR, et al. Multi-kinase RET inhibitor vandetanib combined with mTOR inhibitor everolimus in patients with RET rearranged non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):9035.
- Velcheti V, Hida T, Reckamp KL. Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. Ann Oncol. 2016;27(suppl 6):VI417.
- Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer. 2019;138:124–30.
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–60.
- Gainor JF, Curigliano G, Kim D-W, et al. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15_suppl):9515.
- Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403–10.
- Drilon A, Fu S, Patel MR, et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019;9(3):384–95.
- Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41.
- Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813–9.
- Schöffski P, Elisei R, Müller S, et al. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol. 2012;30 (15_suppl):5508.
- Nair A, Lemery SJ, Yang J, et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin Cancer Res. 2015;21(23):5205–8.
- Brose MS, Nutting CM, Jarzab B, et al; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28.
- Griesinger F, Roeper J, Pöttgen C, et al. Brain metastases in ALK-positive NSCLC - time to adjust current treatment algorithms. Oncotarget. 2018;9(80):35181–94.
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–25.
- Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13(10):1595–601.
- Wang H, Wang Z, Zhang G, et al. Driver genes as predictive indicators of brain metastasis in patients with advanced NSCLC: EGFR, ALK, and RET gene mutations. Cancer Med. 2020;9(2):487–95.
- Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol. 2013;31(31):3987–96.
- Wang H, Tang J, Su Z. YAP confers resistance to vandetanib in medullary thyroid cancer. Biochem Cell Biol. 2020;98(3):443–8.
- Liu X, Shen T, Mooers BHM, et al. Drug resistance profiles of mutations in the RET kinase domain. Br J Pharmacol. 2018;175(17):3504–15.
- Werner RA, Schmid JS, Muegge DO, et al. Prognostic value of serum tumor markers in medullary thyroid cancer patients undergoing vandetanib treatment. Medicine (Baltimore). 2015;94(45):e2016.
- La Pietra V, Sartini S, Botta L, et al. Challenging clinically unresponsive medullary thyroid cancer: discovery and pharmacological activity of novel RET inhibitors. Eur J Med Chem. 2018;150:491–505.
- Drilon A, Rogers E, Zhai D, et al. TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven Cancers. Ann Oncol. 2019;30(suppl_5):v159–v193.
- Cooper M, O'Connor-Semmes R, Reedy BA, et al. First-in-human studies for a selective RET tyrosine kinase inhibitor, GSK3179106, to investigate the safety, tolerability, and pharmacokinetics in healthy volunteers. Clin Pharmacol Drug Dev. 2019;8(2):234–45.
- Schöffski P, Aftimos PG, Massard C, et al. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non-small cell lung cancer and medullary thyroid cancer. J Clin Oncol. 2019;37(15_suppl):TPS3162.
- Alqahtani T, Kumarasamy VM, Huczyński A, Sun D. Salinomycin and its derivatives as potent RET transcriptional inhibitors for the treatment of medullary thyroid carcinoma. Int J Oncol. 2020;56(1):348–58.
- Chrisoulidou A, Mandanas S, Margaritidou E, et al. Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. Onco Targets Ther. 2015;8:2435–42.
- Rosenberg SM, Petrie KJ, Stanton AL, et al. Interventions to enhance adherence to oral anti-neoplastic agents: a scoping review. J Natl Cancer Inst. 2020;112(5):443–65.
- Priya SR, Dravid CS, Digumarti R, Dandekar M. Targeted therapy for medullary thyroid cancer: a review. Front Oncol. 2017;7:238.
- Retevmo (selpercatinib) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2020.
- Yeoh TT, Si P, Chew L. The impact of medication therapy management in older oncology patients. Support Care Cancer. 2013;21(5):1287–93.
- Li Y, Ling L, Zhanyu P. Effect of wellness education on quality of life of patients with non-small cell lung cancer treated with first-line icotinib and on their family caregivers. Integr Cancer Ther. 2019;18:1534735419842373.
- Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5(2):112–9.
- Blueprint Medicines Announces Submission of New Drug Application to FDA for Pralsetinib for the Treatment of Advanced RET Mutant and RET Fusion-Positive Thyroid Cancers [news release]. Cambridge, MA: Blueprint Medicines Corporation. July 1, 2020. http://ir.blueprintmedicines.com/news-releases/news-release-details/blueprint-medicines-announces-submission-new-drug-application.
- Express Scripts. FDA Approved Drugs: June 2020. https://www.express-scripts.com/corporate/articles/fda-approved-drugs-june-2020. Published June 16, 2020.
- S. National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/.
Back to Top