
Expired activity
Please go to the PowerPak
homepage and select a course.
The Role of Immune Checkpoint Inhibitors in the Treatment of Genitourinary Cancers: An Interprofessional Perspective on Guideline Updates
INTRODUCTION TO IMMUNOTHERAPY IN GENITOURINARY CANCERS
Genitourinary (GU) cancers will account for approximately 362,860 new cancer cases and approximately 68,030 deaths in the United States (US) in 2020.[Seigel 2020] Immunotherapy agents have been approved in all GU malignancies, though no immune checkpoint inhibitors (ICI) have been approved for the treatment of prostate cancer. Since these agents differ from chemotherapy and targeted therapies, it is critical for members of the care team to understand the fundamentals of immunotherapy and associated toxicities to ensure that patients are appropriately managed. Patient and caregiver education, clinical assessment, intervention, and interprofessional collaboration will enhance both clinical outcomes and quality of life for GU cancer patients and their loved ones.
SECTION 1: Introduction to Immunotherapy in Genitourinary Cancers
Overview of the current state
Cancer immunotherapy refers broadly to any treatment that utilizes components of the immune system to prevent immune system evasion by tumor cells and to boost immune response against tumors.[Hayes 2020] While the fundamentals of immunotherapy can be traced back to the 19th century with William Coley and his use of bacterial toxins to treat patients with sarcomas, modern options have become increasingly refined.[Zhang 2020] Early examples of immunotherapies for GU cancers include high-dose interleukin 2 (IL-2) and interferon alfa, although their toxicities were evident since their inception and their current role in therapy is limited.[deKernion 1983, Rosenberg 1985] The first modern example of a US Food and Drug Administration (FDA)-approved immune checkpoint inhibitor was the cytotoxic T-lymphocyte antigen-4 (CTLA-4) blocking monoclonal antibody ipilimumab.[FDA 2020A] While early clinical trial results showed an incidence of grade 3-4 immune-mediated adverse events (AEs) of 10-30%, they were typically more manageable than those seen with high-dose IL-2 therapy and acceptable based on durable responses.[Robert 2011]
Subsequently, the programmed death 1 (PD-1) receptor was discovered as a viable target and found to produce durable responses in a variety of tumors in preliminary studies.[Kirkwood 2012] The first two agents that were studied were BMS-936558 (nivolumab) and MK-3475 (pembrolizumab), which showed response rates in advanced cancer ranging from 18-38% depending on the tumor type, the majority of which lasted up to a year. Additionally, grade 3-4 immune-related AEs were generally in the range of 12-14%, thus providing the durable responses seen with previous immune-based therapies with a more manageable safety profile.[Topalian 2012][Hamid 2013] Since then, the treatment of multiple cancer types – including GU cancers – have undergone a paradigm shift as immunotherapies play a more prominent role in treatment algorithms.
Loss of function of the von Hippel–Lindau (VHL) gene leads to dysregulation of growth factors, including vascular endothelial growth factor (VEGF) in clear cell renal cancer, which leads to tumor cell proliferation and tumor growth. Anti-angiogenic therapies have been approved based on their ability to inhibit VEGF, tumor growth, and disease progression, leading to improved disease control and survival in metastatic renal cancer. The ability of anti-VEGF therapies to inhibit dendritic cell maturation, and to promote the accumulation and activation of T-regulatory cells and myeloid-derived stem cells, alters the tumor microenvironment and improves their function as immunosuppressive cells. [Gabrilovich 2012][Lanitis 2015] Anti-VEGF therapies enhance the ability of T cells to extravasate through the endothelium and infiltrate into tumors, and increase immunosuppression of the tumor microenvironment by inducing the accumulation of myeloid-derived suppressor cells and regulatory T cells, providing the rationale for the combination.[Motz 2013][Garje 2020] The combination of anti-VEGF and checkpoint inhibitor therapy has led to increased efficacy leading to the FDA approval of two combination regimens in renal cancer. A prerequisite for successful reversal of tumor-induced immunosuppression is the ability of tumor antigen-specific T cells to activate and infiltrate into tumors.[Allen 2017] Moreover, the combination therapy of immune checkpoint inhibitors with targeted agents such as pembrolizumab or avelumab with axitinib has demonstrated both safety and efficacy (Figure 1).
Figure 1. Immunotherapy combinations and mechanisms of action.
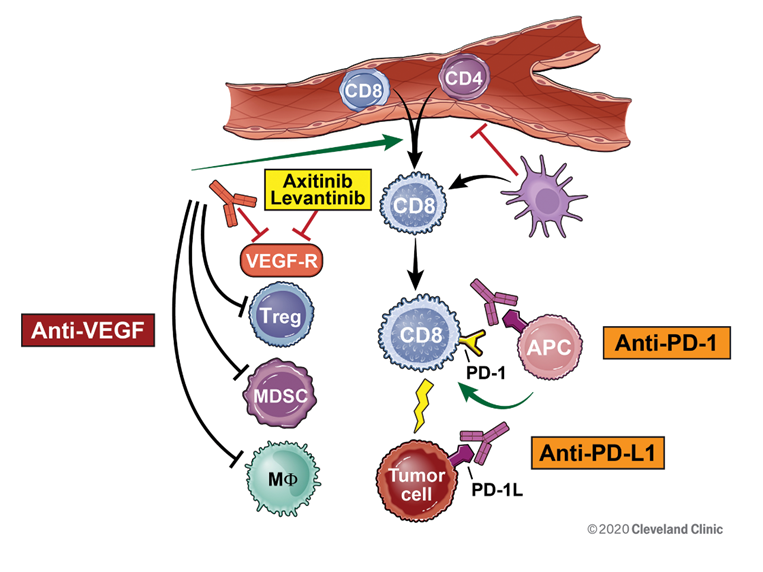
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Immunotherapies that are currently available for treating GU cancers are summarized in Table 1.
| Table 1. Immunotherapy agents used in the treatment of genitourinary cancers. |
| Generic Name |
Trade Name |
Blocks PD-1 |
Blocks PD-L1 |
Blocks CTLA-4 |
| Atezolizumab |
Tecentriq |
|
X |
|
| Avelumab |
Bavencio |
|
X |
|
| Durvalumab |
Imfinzi |
|
X |
|
| Ipilimumab |
Yervoy |
|
|
X |
| Nivolumab |
Opdivo |
X |
|
|
| Pembrolizumab |
Keytruda |
X |
|
|
Barriers in immunotherapy among the clinical care team
Oncology treatment recommendations are changing rapidly as new clinical trial data and longer-term follow-up data are reported. National Comprehensive Cancer Network (NCCN) guidelines are revised frequently to reflect new information regarding treatment outcomes from clinical trials. The Society for Immunotherapy of Cancer (SITC) has published consensus statements on immunotherapy for the treatment of advanced renal cell carcinoma and other cancers.[Rini 2019] Members of the care team must keep abreast of changes in treatment options, oncologic drug approvals, and changes to existing drug labels. Both nivolumab and pembrolizumab had revisions to the dose and frequency of administration, which had significant impacts on treatment planning. The approval to administer pembrolizumab 400 mg every 6 weeks was well-suited to meet the need to minimize the frequency of clinic visits due to the COVID pandemic.
Understanding the differences among mechanisms of action and toxicity profiles is critical as immunotherapy is now being combined with chemotherapy or targeted therapy. Assessment and treatment modification strategies require the clinician to determine if AEs are related to one drug or an overlapping effect of multiple drugs when immunotherapy is given as a combination regimen. Gaps in knowledge regarding toxicity profiles can lead to inappropriate treatment interruption or delay in initiation of appropriate interventions.
Collaboration within the oncology team, and incorporation of interprofessional management of individuals with GU malignancies can improve clinical outcomes. Tumor Boards and Grand Rounds offer the opportunity to share expertise and develop best practices in oncology care. Determining the appropriate treatment and sequence of systemic and local therapies can improve outcomes, and interprofessional management of immune-related adverse events (irAEs) can minimize the severity and duration of toxicities.
Finally, the cost of immune checkpoint inhibitors can impose a significant financial burden on both patients and healthcare institutions if not covered by the patient’s insurer. High co-pays associated with oral targeted therapies can also limit patient adherence, thus posing a significant barrier to achieving optimal care. Pharmacists can help mitigate this by ensuring these medications are being used for FDA-approved or well-evaluated indications, as well as locating resources for copay assistance or free drug replacement to allow patients access to these medications.[ACCC 2016]
Interprofessional Collaboration when managing patients with Genitourinary Cancers
1. How can the members of the care team work together in the clinical decision-making process to improve outcomes in patients with GU cancers?
2. In what ways does the oncology pharmacist’s role complement the roles of other team members such as the oncology nurse, the oncologist, and oncology advanced practice providers?
You can read the transcripts at the link provided at the end of the activity.
|
SECTION 2: Immunotherapy in Renal Cell Cancer
Immunotherapy has changed significantly since the initial approval of Interleukin-2 in 1992. The development of immune checkpoint inhibitor therapy has led to the approval of three regimens as front-line treatment for advanced renal cell carcinoma. Ipilimumab in combination with nivolumab was approved in 2018 for intermediate or poor-risk advanced renal cell carcinoma (RCC). In 2019, the approval of avelumab in combination with axitinib was quickly followed by the approval of pembrolizumab with axitinib for first-line treatment in advanced RCC. These approvals expand treatment options and increase the need for oncology clinicians to gain expertise in the data regarding efficacy and toxicity of these therapeutic strategies. All three of these regimens were approved based on improvement in response rate (RR) and overall survival (OS) compared to sunitinib monotherapy (Table 2). Ongoing long-term follow up and the emergence of new immunotherapy combination data strengthen the body of evidence for the incorporation of immunotherapy in the treatment of renal cell carcinoma.
| Table 2. Phase 3 studies of VEGF targeted therapies in combination with immunotherapies versus sunitinib.[Plimack 2020][Escudier 2019][Motzer 2019][Rini 2019][Motzer 2018] |
| |
Pembrolizumab + Axitinib
vs
Sunitinib |
Avelumab + Axitinib
vs
Sunitinib |
Nivolumab + Ipilimumab
vs
Sunitinib |
| IMDC prognostic risk % |
| Favorable |
31.2 |
21.4 |
23 |
| Intermediate |
56.2 |
61.8 |
61 |
| Poor |
12.6 |
16.2 |
17 |
| Overall Survival |
| Hazard ratio for death |
0.68 |
0.78 |
0.68 |
| P value |
<0.001 |
0.14 |
<0.001 |
| Median progression-free survival, months |
| Immunotherapy + VEGF |
15.4 |
13.8 |
12.4 |
| Sunitinib |
11.1 |
8.4 |
12.3 |
| Overall response rate, % |
60.2 vs 39.9 |
51.4 vs 25.7 |
39 |
| Complete response rate, % |
8.8 vs 3.0 |
3.4 vs 1.8 |
10.2 |
| Median follow-up, months |
24 |
11.6 |
25.2 |
| IDMC, International Metastatic Renal Data Base Consortium |
Non-clear cell renal cell carcinoma
Approximately 25% of patients diagnosed with RCC have a non-clear-cell histology. These kidney cancer subtypes include papillary (15%), chromophobe (5%), and other rare subtypes including medullary carcinoma, collecting duct carcinoma, translocation, and unclassified.[Ahrens 2019] Treatment options include VEGF or mammalian target of rapamycin (mTOR)-targeted therapies and more recently, checkpoint inhibitor therapies. Clinical trials of checkpoint inhibitors in this population are extremely limited. A pooled analysis of forty-three patients with non-clear cell RCC treated with PD-1 and PD-L1 therapies at 8 international institutions demonstrated an overall response rate (ORR) of 19%, with a median time-to-treatment failure of 4.0 months showing the need for additional clinical trials in this patient population.[McKay 2018] Sarcomatoid RCC is a rare and aggressive subtype accounting for approximately 5% of diagnoses, most often presenting in an advanced stage with a very poor prognosis.[Shuch 2012] In the Phase 3 study of pembrolizumab plus axitinib versus sunitinib, a subgroup analysis of patients with sarcomatoid features demonstrated an improved 12-month OS with pembrolizumab plus axitinib compared to sunitinib (83.4% vs. 79.5%) and a progression free survival (PFS) not reached for the combination arm compared to 8.4 months for sunitinib.[Rini 2019]
Risk stratification
The International Metastatic Renal Data Base Consortium (IMDC) criteria resulted from the review of over 75,000 patients with metastatic RCC to identify characteristics that define three prognostic risk groups.[Heng 2009] Lab and clinical criteria distinguish between favorable, intermediate, and poor risk groups (Table 3). Updated survival data demonstrated a 43-month overall survival for those in the favorable risk group, 23-month for those in the intermediate risk group, and 8-month survival for those in the poor risk group.[Heng 2013]
| Table 3. International Metastatic Renal Data Base Consortium (IMDC) prognostic factors.[Heng 2009] |
|
Clinical factors:
Low Karnofsky performance status (<80%)
Time from diagnosis to treatment <1 year
|
Favorable risk group: 0 factors
Intermediate risk group: 1-2 factors
Poor risk group: 3 or more factors
|
|
Laboratory factors:
Low hemoglobin (<LLN)
High corrected calcium (>ULN)
High neutrophil count (>ULN)
High platelet count (>ULN)
|
| LLN, lower limit of normal; ULN, upper limit of normal. |
Results from the clinical trials of combination regimens including immunotherapies versus a targeted VEGF inhibitor (Table 2) formed the basis for the most recent NCCN recommendations for metastatic renal cancer (Table 4).[NCCN Kidney 2020]
NCCN 2019/2020 updates and treatment recommendations
| Table 4. NCCN guidelines for first-line therapy for clear cell histology.[NCCN Kidney 2020] |
| Risk |
Preferred |
Other Recommended |
Useful in Certain Situations |
| Favorable |
Axitinib + Pembrolizumab |
Ipilimumab + Nivolumab |
Active surveillance |
| Pazopanib |
Axitinib + Avelumab |
Axitinib |
| Sunitinib |
Cabozantinib |
High-dose IL-2 |
| Intermediate/Poor |
Ipilimumab + Nivolumab |
Pazopanib |
Axitinib |
| Axitinib + Pembrolizumab |
Sunitinib |
High-dose IL-2 |
| Cabozantinib |
Axitinib + Avelumab |
Temsirolimus |
| IL, interleukin |
| Table 5. NCCN guidelines for front-line therapy for non-clear cell advanced RCC.[NCCN Kidney 2020] |
| Preferred |
Other Recommended |
Useful in Certain Situations |
| Clinical Trial |
Cabozantinib |
Axitinib |
| Everolimus |
Bevacizumab + Erlotinib |
| |
Nivolumab |
| Sunitinib |
Lenvatinib + Everolimus |
Pazopanib |
| |
Bevacizumab + Everolimus |
| |
Temsirolimus |
| Table 6. NCCN guidelines subsequent therapy for clear cell histology.[NCCN Kidney 2020] |
| Preferred |
Other Recommended |
Useful in Certain Situations |
| Cabozantinib |
Axitinib |
Bevacizumab |
| Nivolumab |
Lenvatinib + Everolimus |
Sorafenib |
| Ipilimumab + Nivolumab |
Axitinib + Pembrolizumab |
High-dose IL-2 |
| |
Everolimus |
Temsirolimus |
| |
Pazopanib |
|
| |
Sunitinib |
|
| |
Axitinib + Avelumab |
|
| IL, interleukin |
Resources
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Kidney Cancer Version 2.2020 — August 5, 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
NCCN Guidelines for Patients Kidney Cancer Version 2020. Available at https://NCCN.org/patients
CASE 1: GLENN: RENAL CANCER
Glenn, a 68-year-old male, is referred to a medical oncologist for the management of new lung metastasis 9 months following a radical nephrectomy for clear cell renal carcinoma. He has a history of hypertension which is well-controlled, and lab tests demonstrate mild anemia and mild chronic kidney disease results from his prior nephrectomy.
CASE 1 QUESTIONS
PAUSE AND REFLECT: Assess case questions based on content thus far, then listen to faculty commentary and read the transcripts at the link provided at the end of the activity.
- Is Glenn in the favorable or intermediate risk group according to the International Metastatic Renal Data Base Consortium (IMDC) criteria?
- Based on the NCCN guidelines, what immunotherapy treatment options should be considered?
- What would you want to discuss with him regarding potential side effects of treatment?
|
SECTION 3: Immunotherapy in Bladder Cancer
Immune checkpoint inhibitors established their role in the treatment of locally-advanced and metastatic urothelial carcinoma (UC) in 2016 when the FDA approved atezolizumab for the treatment of patients with disease progression during or following platinum-based chemotherapy, or progression within 12 months of neoadjuvant or adjuvant platinum-based therapy. Since then, a total of five immunotherapy agents have been approved for the treatment of UC, each with at least one indication.[FDA 2020A] Similarly, the NCCN has also made several updates regarding immunotherapy in its UC treatment algorithm. A summary of all NCCN recommended immunotherapy agents is included in Table 7 along with caveats for use when appropriate. The most recent additions to the algorithm have been for the use of pembrolizumab in non-muscle invasive tumors and for avelumab as maintenance therapy.[NCCN Bladder 2020]
| Table 7. NCCN recommended immunotherapy agents for bladder cancer.[NCCN Bladder 2020] |
| Medication |
Non-muscle invasive, high risk, BCG-unresponsive |
First-line, locally advanced or metastatic |
Maintenance, locally advanced or metastatic* |
Subsequent line, locally advanced or metastatic |
| Atezolizumab |
|
X† |
|
X |
| Avelumab |
|
|
X |
X |
| Durvalumab |
|
|
|
X |
| Nivolumab |
|
|
|
X |
| Pembrolizumab |
X |
X† |
|
X |
*For patients who have not progressed after first-line platinum therapy
†In cisplatin-ineligible patients with tumor-infiltrating cell PD-L1 >5%, or platinum-ineligible regardless of PD-L1 expression |
An important update in 2018 was the addition of the caveats to the use of atezolizumab or pembrolizumab as first-line therapy for locally-advanced or metastatic UC, which was based on revised FDA approvals. In the IMvigor130 study, patients with untreated, locally-advanced or metastatic UC were randomized to one of three treatment groups: platinum-based therapy + atezolizumab (Group A); atezolizumab monotherapy (Group B); or platinum-based therapy + placebo (Group C). Similar to previous studies, the investigators conducted subgroup analyses of patients based on tumor-infiltrating immune cells (IC) PD-L1 expression. In 2018, an interim analysis showed decreased survival in the atezolizumab monotherapy group compared to the chemotherapy group in patients with <5% PD-L1 /expression, prompting the FDA to revise its previous approval.[Suzman 2019] The updated labeling required IC PD-L1 >5% for use in the first-line setting for cisplatin-ineligible patients, although it could be used in patients who were completely ineligible for platinum-therapy regardless of PD-L1 expression.[FDA 2018]
In the similarly designed KEYNOTE-361 study, patients with untreated, locally-advanced or metastatic UC were randomized to: platinum-based therapy + pembrolizumab (Group A); pembrolizumab monotherapy (Group B); or platinum-based therapy + placebo (Group C). Patient were stratified by PD-L1 expression as determined by Combined Positive Score (CPS), which reflects expression by both the tumor and immune cells. After a median follow-up of 7.8 months, the ORR was 21% in patients with CPS <10 or unknown, and 47% in patients with CPS >10. Additionally, the data monitoring committee conducted an interim analysis and found decreased survival in the pembrolizumab monotherapy arm compared to chemotherapy in patients with low PD-L1 expression.[Suzman 2019] As a result, the FDA updated pembrolizumab’s labeling to require PD-L1 CPS >10 prior to use in the first-line setting for cisplatin-ineligible patients, but could be used regardless of PD-L1 status in patients who were completely ineligible for platinum therapy.[FDA 2018]
In January 2020, pembrolizumab was approved for treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in-situ who are ineligible for or elect to not undergo surgery.[FDA 2020B] The approval was granted based on results of KEYNOTE-057, in which patients were treated with pembrolizumab for up to 24 months or until recurrence, progression, or unacceptable toxicity. The primary endpoint, complete response rate (CRR), was reached in 38.8% of patients at 3 months. After a median follow-up of 14 months, 72.5% of patients who achieved a 3-month complete response maintained their response, and none of these patients experienced progression to muscle-invasive or metastatic disease.[Balar 2019] Additionally, the study investigators presented an update after a median follow-up of 28.4 months, which showed a CRR of 40.6%. Similar to the initial results, no patients experienced progression to muscle-invasive or metastatic disease while receiving study drug. While the median PFS and OS were not reached, the PFS and OS at 12 months were 82.7% and 97.9%, respectively.[Balar 2020][Vucky 2020] Based on this, the NCCN provided a category 2A recommendation for the use of pembrolizumab in this patient population, which is the only recommendation it gives for an immune checkpoint inhibitor in the non-metastatic UC setting.[NCCN Bladder 2020]
Most recently, avelumab was FDA-approved in June 2020 as maintenance therapy in patients with locally-advanced or metastatic disease that has not progressed after first-line platinum-based therapy.[FDA 2020C] In the Phase 3 JAVELIN Bladder 100 trial, 700 patients who had a response or stable disease after 4-6 cycles of platinum-based therapy received avelumab with best supportive care (BSC) or BSC alone. The median OS for avelumab was 21.4 months compared to 14.3 months in the BSC group (HR=0.69; 95% CI 0.56-0.85, p=0.0005). This benefit was preserved regardless of whether the patient had a response or stable disease at the end of chemotherapy, or if they had visceral or non-visceral disease when initiating chemotherapy.[Powles 2020] In the most recent NCCN guideline update in July 2020, avelumab is listed with a category 2A recommendation as maintenance therapy for these patients.[NCCN Bladder 2020]
Several other ongoing trials are evaluating the use of immunotherapy in different aspects of UC and, based on most recent results, may become part of the next guideline update. In the SWOG S1605 Phase 2 trial, patients with BCG-unresponsive NMIBC who were unfit for or refused radical cystectomy were treated with atezolizumab for one year. In the subgroup of patients who were cisplatin-eligible, the 3-month and 6-month CRR were 41.1% and 26%, respectively.[Black 2020] The 3-month CRR is similar to that reported in the KEYNOTE-057 study, indicating a potential long-term benefit of atezolizumab in this same patient population.
Similar to the JAVELIN Bladder 100 trial, Galsky et al. conducted a placebo-controlled study evaluating the effect of “switch maintenance” with pembrolizumab for up to two years in patients who did not progress after first-line platinum therapy for metastatic UC. A total of 55 patients were randomized to pembrolizumab and 53 to the placebo arm, with an OR of 23% and 10%, respectively. The PFS was significantly longer with pembrolizumab compared to placebo (5.4 vs 3 months; HR=0.65, p=0.039), but the median OS was similar at 22 months and 18.7 months.[Galsky 2020] While the OS results did not replicate the difference seen in the JAVELIN Bladder 100 trial, this may be due in part to the significantly smaller sample size; thus, further prospective studies are necessary to validate these results.
Study EV-103 is investigating the first-line combination of pembrolizumab and enfortumab vedotin for patients with locally-advanced or metastatic UC. Enfortumab vedotin is an antibody drug conjugate (ADC) that targets overexpression of Nectin-4 on UC cells to deliver MMAE, a potent microtubule inhibitor.[Rosenberg 2020] It has already been FDA approved as subsequent monotherapy for locally-advanced or metastatic UC, and received an NCCN category 2A recommendation for the same.[FDA 2020A][NCCN Bladder 2020] After a median follow-up of 11.5 months, the cohort of cisplatin-ineligible patients demonstrated an ORR of 73.3% and disease control rate of 93.3%. Additionally, the median PFS and OS were 12.3 months and not reached, respectively.[Rosenberg 2020] Despite the relatively small sample size of 45 patients, this study may prompt NCCN consideration for inclusion in the next guideline review.
Resource
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Bladder Cancer. Version 5.2020 — May 12, 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
CASE 2: SANDRA: BLADDER CANCER
Sandra, a 70-year-old female with a 40-year smoking history, presents to her urologist with complaints of increased urinary frequency and mild hematuria. A CT scan of the abdomen and pelvis, CT urography, and transurethral resection of the bladder (TURBT) are performed, which confirm the diagnosis of high-grade NMIBC with urothelial carcinoma in-situ (Tis). Post-TURBT, she undergoes adjuvant therapy with intravesical BCG; however, follow-up cystoscopy shows persistent disease at 3 months. She undergoes repeat TURBT and receives a dose of intravesical gemcitabine within 24 hours. A discussion is had with her regarding the possibility of radical cystectomy, which she declined.
CASE 2 QUESTIONS
PAUSE AND REFLECT: Assess case questions based on content thus far, then listen to faculty commentary and read the transcripts at the link provided at the end of the activity.
- Based on current NCCN guidelines, which immunotherapy agent(s) would be most appropriate to consider for Sandra?
- She declines immunotherapy at this time and instead is agreeable to adjuvant induction therapy with gemcitabine. If she progresses to locally-advanced or metastatic disease, which immunotherapy agent(s) would be appropriate per NCCN guidelines as first-line treatment? Are there any factors to consider before selecting an immunotherapy for first-line treatment?
- Her disease progresses, with both hepatic and lung metastases present. She still has normal renal function, so she is started on platinum-based chemotherapy and achieves a partial response after 6 cycles. Which immunotherapy agent(s) would be NCCN recommended to initiate as maintenance therapy in this patient?
|
SECTION 4: Immunotherapy in Prostate Cancer
In contrast to the widespread use of immune checkpoint inhibitors in renal cell and bladder malignancies, pembrolizumab is the only immune checkpoint inhibitor included in the NCCN guidelines for prostate cancer.[NCCN Prostate 2020] Pembrolizumab obtained FDA approval for this indication based on the culmination of results from five different studies examining its effect in patients with microsatellite stability high or mismatch repair deficient (MSI-H/dMMR) solid tumors that have progressed after at least one therapy. Of the total 149 patients across the five studies, the ORR was 39.6%, although 90 of the patients included had colorectal cancer.[Marcus 2019] More recently, the Phase 2 KEYNOTE-158 study evaluated pembrolizumab specifically in patients with metastatic non-colorectal MSI-H/dMMR solid tumors. The ORR was 34.3%, with a median PFS and OS of 4.1 months and 23.5 months, respectively. However, only six of the 233 evaluable patients in the study had prostate cancer, thus limiting the generalizability of these results to prostate cancer specifically.[Marabelle 2020] Based on the limited data, the NCCN provided a category 2B recommendation for use in patients with metastatic MSI-H/dMMR tumors who have progressed on at least one line of therapy.[NCCN Prostate 2020]
More recent data suggest the potential benefit of combination therapy that includes PD-1/PD-L1 inhibitors for metastatic prostate cancer. The combination of pembrolizumab with an anti-androgen therapy, enzalutamide, was studied in a Phase 2, single-arm study of patients with metastatic prostate cancer that progressed on enzalutamide alone. Of 28 patients enrolled in the study, 18% achieved a prostate specific antigen (PSA) decline of at least 50%, and the ORR was 25%.[Graff 2020]
The KEYNOTE-365 study is a multi-cohort, Phase 1/2 study of pembrolizumab in combination with different agents for the treatment of metastatic castration-resistant prostate cancer (mCRPC) that have received at least one prior therapy and relapsed within 6 months of study screening. Results for multiple cohorts of this study were reported at the American Society of Clinical Oncology (ASCO) 2020 annual meeting; a summary of results are presented in Table 8. Phase 3 confirmation of these results are currently under investigation for cohorts A, B, and C in the KEYLYNK-010, KEYNOTE-921, and KEYNOTE-641 studies, respectively.
| Table 8. KEYNOTE-365 results by cohort. |
| |
Prior Therapy |
Study Regimen |
PSA response rate* |
Median rPFS |
Median OS |
| Cohort A [Yu 2020] |
Docetaxel; may have received 1 other chemotherapy or <2 second generation anti-androgen agents |
Pembrolizumab + Olaparib |
9% |
4.3 months |
14.4 months |
| Cohort B [Sridhar 2020][ Kolinsky 2020] |
Enzalutamide or abiraterone; no prior chemotherapy |
Pembrolizumab + Docetaxel + Prednisone |
28% |
8.3 months |
20.4 months |
| Cohort C [Conter 2020][Berry 2020] |
Abiraterone; no prior chemotherapy |
Pembrolizumab + Enzalutamide |
22% |
6.1 months |
20.4 months |
| *Defined as a decrease >50%; PSA, prostate specific antigen; rPFS, radiographic progression-free survival |
Additionally, results from cohorts 4 and 5 from the KEYNOTE-199 study of the combination of pembrolizumab and enzalutamide were presented at the ASCO 2020 meeting. In this study, patients were included if they progressed on enzalutamide after a clinically meaningful response to enzalutamide and had either RECIST measurable disease (cohort 4) or bone-predominant disease (cohort 5). Median time to prostate specific antigen (PSA) progression and median PFS was 4 months in both cohorts, whereas the median OS was not reached at 19 months in cohorts 4 and 5, respectively.[Antonarakis 2020][Hoimes 2020]
Studies with other PD-1 or PD-L1 inhibitors are limited. In a Phase 2, open-label trial, patients with mCRPC were treated with a combination of durvalumab and olaparib, an oral poly-ADP ribose polymerase (PARP) inhibitor. Of 17 patients in the trial, the median radiographic PFS was 16.1 months, with a higher 12-month PFS in patients with DNA damage repair gene alterations compared to those without them (83.3% vs 36.4%, p=0.031).[Karzai 2018] As part of the multi-cohort, Phase 2 NCI-MATCH trial, Azad et al. reported the results of patients with MMR-deficient, non-colorectal, relapsed or refractory solid tumors that were treated with nivolumab. A total of 42 patients were enrolled, of which 5 of the patients had prostate cancer. The ORR was 36%, with a 12-month PFS of 46.2% and median OS of 17.3 months.[Azad 2020]
Despite this encouraging early data, pembrolizumab remains the only immune checkpoint inhibitor recommended in the NCCN guidelines, and only as subsequent therapy for MSI-H/dMMR tumors. The mainstay of therapy for prostate cancer remains the use of androgen deprivation therapy (ADT) and radiation therapy, depending on the patient’s life expectancy and stage of disease. A full review of prostate cancer treatment guidelines is beyond the scope of this learning activity; the reader is encouraged to review the NCCN guidelines for further details. If a provider and patient would like to pursue the use of an immune checkpoint inhibitor outside of this context, they should strongly consider enrollment in a clinical trial, as the NCCN continually promotes this throughout its guidelines.[NCCN Prostate 2020]
Resources
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Prostate Cancer. Version 2.2020 — May 21, 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
NCCN Guidelines for Patients.® Prostate Cancer. Available at https://www.nccn.org/patients/guidelines/content/PDF/prostate-patient.pdf.
CASE 3: WILLIAM: PROSTATE CANCER
William, a 75-year-old male, presents to his clinician complaining of difficulty voiding urine and pain in his lower back. On physical exam, the patient has a prostate mass, and biopsy results indicate necrosis and primary Gleason pattern 5, suggestive of very high-risk disease. A CT of the abdomen and pelvis is performed, as well as a CT of his lumbar spine, both of which indicate metastatic spread. He is diagnosed with Stage IV prostate cancer and would like to begin therapy as soon as possible.
CASE 3 QUESTIONS
PAUSE AND REFLECT: Assess case based on content thus far, then listen to faculty commentary and read the transcripts at the link provided at the end of the activity.
- According to NCCN guidelines, what immune checkpoint inhibitor(s) would be appropriate for this patient as first-line therapy?
- William is started on androgen deprivation therapy with an LHRH-antagonist, degarelix. After 1 year, he becomes resistant to initial therapy and is started on enzalutamide for his CRPC. Based on current NCCN guidelines, which immune checkpoint inhibitor(s) could be combined with enzalutamide in this setting?
- After an additional year, he is no longer responding to enzalutamide. Based on current NCCN guidelines, what genetic testing should be performed prior to initiating an immune checkpoint inhibitor in this patient?
|
SECTION 5: Safety and Adverse Events
Immune checkpoint inhibitors are associated with unique AEs that are related to the increased activity of the immune system with treatment. Immune-related adverse events (irAEs) can affect multiple organs of the body including skin, gastrointestinal tract, endocrine system, liver, lung, nervous systems, and musculoskeletal systems (Figure 2). Since these agents differ from traditional chemotherapy, it is critical for nurses to understand the fundamentals of immunotherapy and associated toxicities to ensure that patients are treated effectively and safely. Many of the toxicities are driven by the same immunologic mechanism responsible for therapeutic effects leading to inflammatory adverse events, often referred to as ‘immune-related adverse events’ or irAEs.[Postow 2018][Puzanov 2017.]
Figure 2. Immune-related adverse events and systems affected.
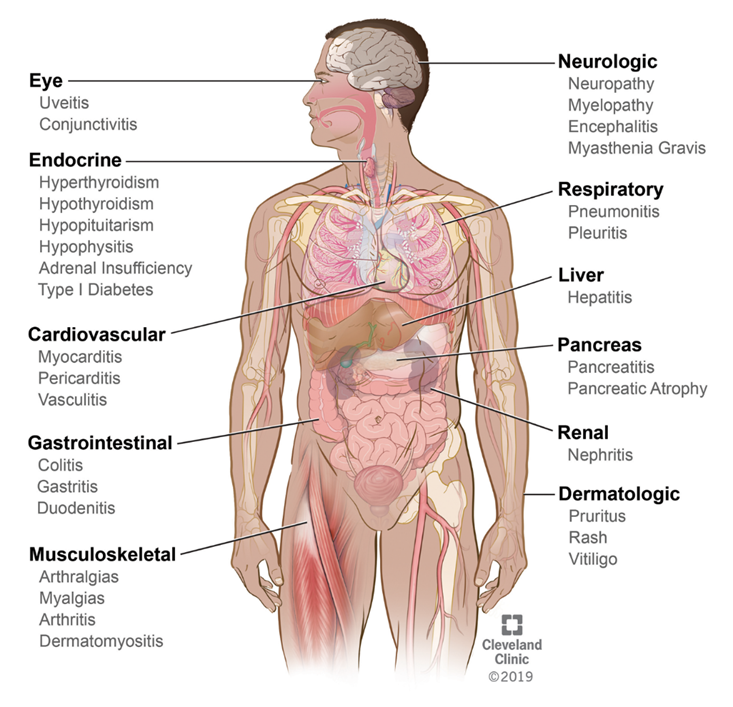
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Toxicity assessment with immunotherapy and chemotherapy combinations
The combination of immunotherapy and chemotherapy has been shown to improve survival outcomes, however, clinicians need to balance toxicity risk and the potential for discontinuation in an individual patient. A meta-analysis of studies of combinations of immunotherapy and chemotherapy in the treatment of solid tumors showed an increased risk of grade 3/4 AEs and higher rates of treatment discontinuation with the combinations compared to monotherapy in different solid tumors. Importantly, however, the combination did not appear to be associated with an increase in fatal adverse events (deaths).[Carretero-Gonzalez 2019]
Table 9 summarizes some important differences in features of chemotherapy and immunotherapy. Figure 3 shows where toxicities differ and overlap when oxaliplatin and 5-fluorouracil (5-FU) chemotherapy are combined with atezolizumab immunotherapy.
| Table 9. Features of adverse events with chemotherapy and immunotherapy. |
| Chemotherapy AEs |
Immunotherapy AEs |
| Almost guaranteed |
May never occur |
| Dose and schedule dependent |
Dose and schedule independent |
| Timing based on drug and schedule |
Can occur anytime |
| May need short course of treatment to resolve |
Need treatment to resolve |
| Type and timing of AE varies greatly |
| Source: Laura Wood, RN, MSN, OCN |
Figure 3. Overlapping toxicities with oxaliplatin, 5-FU, and atezolizumab (immunotherapy).
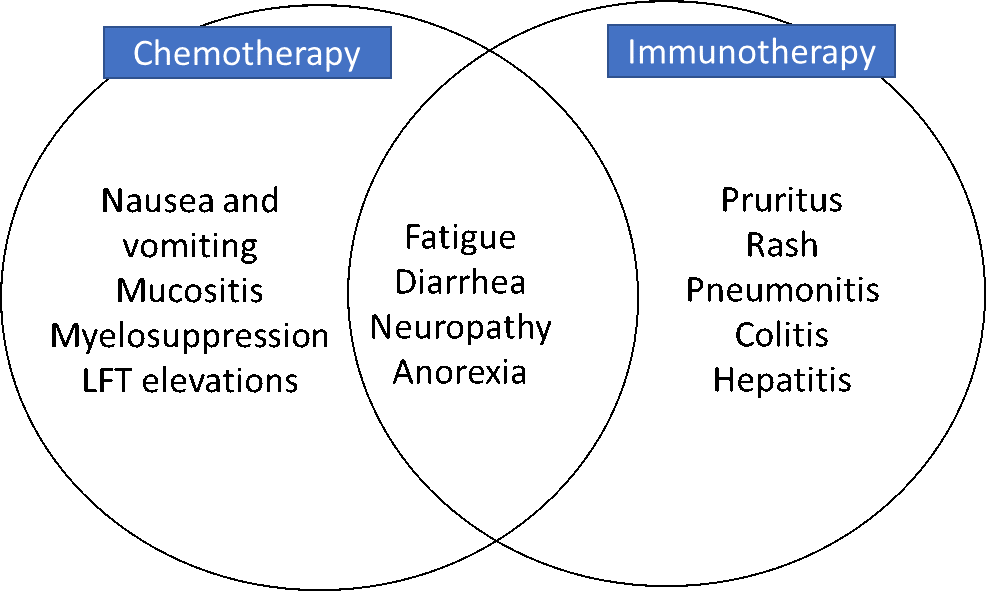
Source: Laura Wood, RN, MSN, OCN
Interprofessional considerations for managing irAEs
Effective management of side effects is crucial to the success of immunotherapy, whether given as a single agent or in combination with chemotherapy or targeted therapy. Education involving patients and their caregivers should be an ongoing process, from the initial discussion regarding treatment options through long-term follow up and survivorship care. While most irAEs are mild to moderate, some can be severe with a transient escalation in severity while others present at a severe or life-threatening level. Oncology health care providers should provide consistent education developed with team input involving both the nurse or advanced practice provider and the pharmacist.[Wood 2019] Important consideration must be given by all clinicians based on the understanding that irAEs can occur in any organ at any time during or following the completion of therapy requiring ongoing assessment and intervention.[Esfahani 2019]
A key role for pharmacists is complementing the nurse’s patient education by reinforcing the prompt recognition and reporting of irAEs and to whom they should report. As patients are often concerned with therapy interruption if they report side effects, pharmacists should clarify that irAEs are generally manageable and immunotherapy can be continued as long as they are caught early.[Medina 2020] While certain toxicities may overlap with targeted therapies and immune checkpoint inhibitors (e.g., fatigue, hepatotoxicity), toxicities from targeted therapies generally abate after discontinuation, which thus presents holding the targeted agent as a strategy to help distinguish the underlying cause. Additionally, a small study regarding pharmacist-led education and monitoring of patients receiving immune checkpoint inhibitors demonstrated improved education and outcomes by referring patients and making appropriate recommendations.[Bosworth 2018] Where allowed by state regulations, pharmacists can form collaborative practice agreements with physicians to order appropriate laboratory tests and initiate medications for supportive care.[Medina 2020]
The NCCN and ASCO have released a joint guideline for the management of irAEs, in which they emphasize steroids as the mainstay of treatment for most irAEs and they must be tapered over >4 weeks.[Brahmer 2018] The guidelines recommend initial treatment with prednisone 0.5-1 mg/kg for mild to moderate symptoms, with an increase to 1-2 mg/kg if patients fail to respond within 2-3 days. Pharmacists can play a role here in recommending appropriate dosing of steroids and timing of dose reductions to ensure patients’ symptoms do not recur. For steroid-refractory immune-mediated colitis or inflammatory arthritis, the tumor necrosis factor (TNF)-alpha inhibitor infliximab can be used. Other options for steroid-refractory irAEs depend on the specific organ effected, but may include vedolizumab, immune globulins, or mycophenolate.[NCCN Toxicities 2019] Pharmacists are well poised to recommend which therapies to use, dosing, and appropriate monitoring plans (e.g., trough levels with mycophenolate) after treatment.[Medina 2020]
Resources
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:17:1714-1768. Available at https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2017.77.6385.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Management of Immunotherapy-Related Toxicities. Version 1.2020 — December 16, 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.
NCCN Guidelines for Patients.® Immunotherapy Side Effects: Immune Checkpoint Inhibitors. Available at https://www.nccn.org/patients/guidelines/content/PDF/immunotherapy-se-ici-patient.pdf
Wood LS, Moldawer NP, Lewis C. Immune checkpoint inhibitor therapy: key principles when educating patients. Clin J Oncol Nurs. 2019;23(3):271-280. doi:10.1188/19.CJON.271-280.
CASE 4: LEE: IMMUNE-RELATED ADVERSE EVENTS
Lee, a 61-year-old male, has been receiving pembrolizumab plus axitinib for 5 months with mild fatigue, and hypertension controlled with amlodipine 10 mg daily and lisinopril 10 mg (added by his nephrologist for HTN and CKD). The patient calls the oncology office to report multiple episodes of loose stool which started 5 days ago.
CASE 4 QUESTIONS
PAUSE AND REFLECT: Assess case questions based on content thus far, then listen to faculty commentary and read the transcripts at the link provided at the end of the activity.
- What questions should the oncology provider ask Lee relative to the report of diarrhea for 5 days?
- What grade is the diarrhea based on CTCAE v. 5?
- What is the appropriate action to take for the diarrhea?
- What is the appropriate follow up for the oncology provider based on the diarrhea?
|
SUMMARY
Results from clinical trials have led to increased efficacy and improved survival for patients with GU malignancies. Improved clinical outcomes and improvements in quality of life for both patients and their loved ones requires care provided by an interprofessional team as collaboration among members of the oncology care team is necessary to optimize outcomes. For all members of the team to maximize their contributions, clinicians will have to continue to enhance their knowledge and skills regarding the latest guidelines and treatment.
REFERENCES
All references accessed as of August 24, 2020.
- Ahrens M, Scheich S, Hartmann A, et al. Non-clear cell renal cell carcinoma-pathology and treatment options. Oncol Res Treatment. 2019;42:128-135.
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulated tumor immunity through HEV formation. Sci Transl Med. 2017;9:1-13.
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395-405.
- Association of Community Cancer Centers. The oncology pharmacist’s role in immuno-oncology. May 3, 2016. Available at https://www.accc-cancer.org/home/learn/resource-detail/The-Oncology-Pharmacists-Role-in-Immuno-oncology.
- Azad NS, Gray RJ, Overman MJ, et al. Nivolumab Is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH (EAY131) study. J Clin Oncol. 2020;38(3):214-222.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab (pembro) for the treatment of patients with Bacillus Calmette-Guerin (BCG) unresponsive, high-risk (HR) non-muscle invasive bladder cancer (NMIBC): over two years follow-up of KEYNOTE-057. J Clin Oncol. 38;15s, 2020 (suppl; abstr 5041).
- Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: phase II trial of pembrolizumab (pembro) for patients (pts) with high-risk (HR) non-muscle invasive bladder cancer (NMIBC) unresponsive to bacillus Calmette-guerin (BCG). J Clin Oncol. 2019;37(7_suppl):350.
- Berry WR, Fong PC, Piulats JM, et al. KEYNOTE-365 cohort C updated results: Pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38:6(suppl):102.
- Black PC, Tangen C, Singh P, et al. Phase II Trial of Atezolizumab in BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: SWOG S1605. 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020.
- Bosworth T. Tapering protocols needed to manage checkpoint inhibitor AEs. Updated May 16, 2018. Available at https://www.clinicaloncology.com/Current-Practice/Article/04-15/Tapering-Protocols-Needed-To-Manage-Checkpoint-Inhibitor-AEs-/48704.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:17:1714-1768.
- Carretero-González A, Lora D, Ghanem I, et al. Comparative safety analysis of immunotherapy combined with chemotherapy versus monotherapy in solid tumors: a meta-analysis of randomized clinical trials. 2019;10(35):3294-3301. Published 2019 May 14.
- Conter HJ, Shore ND, Berry WR, et al. Pembrolizumab (pembro) plus enzalutamide (enza) in patients (pts) with abiraterone acetate (abi)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort C efficacy, safety, and biomarker results. J Clin Oncol. 2020;38(15s_suppl):abstr 5545.
- deKernion JB, Sarna G, Figlin R, et al. The treatment of renal cell carcinoma with leukocyte alpha-interferon. J Urol. 1983;130(6):1063-1066.
- Escudier B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N Engl J Med. 2019;380(12):1176-1178. doi:10.1056/NEJMe1900887.
- Esfahani K, Meti N, Miller WH, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. Can Med Assoc J. 2019;191:E40-6.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Reviews Immunol. 2012;12:253-268.
- Galsky MD, Mortazavi A, Milowsky MI, et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J Clin Oncol. 2020;38:16: 797-806.
- Garje R, An J, Greco A, Vaddepally RK, Zakharia Y. The future of immunotherapy-based combination therapy in metastatic renal cell carcinoma. Cancers (Basel). 2020;12(1):143. Published 2020 Jan 7.
- Graff JN, Beer TM, Alumkal JJ, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer. 2020;8(2):e000642.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma [published correction appears in N Engl J Med. 2018 Nov 29;379(22):2185]. N Engl J Med. 2013;369(2):134-144.
- Hayes C. Cellular immunotherapies for cancer. Ir J Med Sci. 2020;1-17.
- Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141-148.
- Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794-5799.
- Hoimes CJ, Graff JN, Tagawa ST, et al. KEYNOTE-199 cohorts (C) 4 and 5: Phase II study of pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(15s_suppl):abstr 5543.
- Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309-335.
- Kolinsky MP, Gravis G, Mourey L, et al. KEYNOTE-365 cohort B updated results: Pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38:6(suppl):103.
- Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. 2015;33:55-63. doi:10.1016/j.coi.2015.01.011.
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1-10.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753-3758.
- McKay RR, Bosse D, Xie W, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res. 2018; 6(7)758-765.
- Medina P, Jeffers KD, Trinh VA, et al. The role of pharmacists in managing adverse effects related to immune checkpoint inhibitor therapy. J Pharm Pract. 2020;33(3):338-349.
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61-73.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103-1115.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370-1385.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277-1290.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Kidney Cancer Version 2.2020 — August 5, 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Bladder Cancer. Version 5.2020 — May 12, 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Bladder Cancer. Version 5.2020 — May 12, 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Prostate Cancer. Version 2.2020 — May 21, 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- Plimack ER, Rini BI, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): Updated analysis of KEYNOTE-426. J Clin Oncol. 2020;38(suppl 15):abstract 5001.
- Postow A, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018:378:158-168.
- Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN bladder 100 phase III interim analysis. J Clin Oncol. 2020;38;(18_suppl):LBA1.
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Group. J Immunother Cancer. 2017:5:1-28.
- Rini BI, Battle D, Figlin RA, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. 2019;7:354-374.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116-1127.
- Rini BI, Plimick ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for metastatic renal cell carcinoma: Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgorups of the phase 3 KEYNOTE-426 study. J Clin Oncol. 2019;37(suppl 15):abstract 4500.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517-2526.
- Rosenberg JE. Study EV-103: Preliminary Durability Results of Enfortumab Vedotin Plus Pembrolizumab for Locally Advanced or Metastatic Urothelial Carcinoma. 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California.
- Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485-1492.
- Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: A comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46-54.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70: 7-30.
- Sridhar SS, Kolinsky MP, Gravis G, et al. Pembrolizumab (pembro) plus docetaxel and prednisone in patietns (pts) with abiraterone acetate (abi) or enzalutamide (enza)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort B efficacy, safety, and biomarker results. J Clin Oncol. 2020;38(15s_suppl); abstr 5550.
- Suzman DL, Agrawal S, Ning YM, et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist. 2019;24(4):563-569.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
- United States Food and Drug Administration (FDA). FDA approves avelumab for urothelial carcinoma maintenance treatment. June 30, 2020. Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment. [FDA 2020C]
- United States Food and Drug Administration (FDA). FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. January 8, 2020. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. [FDA 2020B]
- United States Food and Drug Administration (FDA). FDA Updates prescribing information for Keytruda and Tecentriq. Updated August 8, 2018. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-updates-prescribing-information-keytruda-and-tecentriq.
- United States Food and Drug Administration (FDA). Hematology/oncology (cancer) approvals & safety notifications. Updated August 6, 2020. Available at https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm.
- United States Food and Drug Administration (FDA). Hematology/oncology (cancer) approvals & safety notifications. Updated August 6, 2020. Available at https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. [FDA 2020A]
- Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020;38(23):2658-2666.
- Wood LS, Moldawer NP, Lewis C. Immune Checkpoint inhibitor therapy: key principles when educating patients. Clin J Oncol Nurs. 2019;23(3):271-280.
- Yu EY, Rodriguez JMMP, Gravis G, et al. Pembrolizumab (pembro) plus olaparib in patients (pts) with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort A efficacy, safety, and biomarker results. J Clin Oncol. 2020;38(15s-suppl):abstr 5544.
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807-821.
Back to Top