
Expired activity
Please go to the PowerPak
homepage and select a course.
Severe Asthma Management: Addressing Emerging Evidence Regarding Phenotypes and Biomarkers
SECTION 1: Severe Asthma Overview
Asthma is a common respiratory disease, affecting both children and adults, and is usually characterized by chronic airway inflammation. It is defined by respiratory symptoms such as wheeze, shortness of breath, chest tightness and/or cough, and by variable expiratory airflow limitation. Symptoms vary over time and in intensity, and are generally triggered by factors such as exercise, allergens such as pet dander and pollen, or irritants like tobacco smoke exposure, upper respiratory tract infections, or changes in weather.(GINA 2019) Patients can experience episodic flare ups of asthma that can be life-threatening. Asthma can also be associated with bronchial hyperresponsiveness. Asthma is heterogeneous in nature. Individuals with asthma can vary widely in clinical presentation, severity, and pathobiology. This heterogeneity can make it difficult to identify and treat severe asthma.
In the United States (US), approximately 9% of children and 7% of adults currently have asthma, and the prevalence of asthma increased from 2001 through 2009.(CDC 2016; Moorman 2014) Asthma has been increasing since the early 1980’s in all age, sex, and racial groups.(CDC.gov) Asthma affects between 1% and 18% of the world’s population and it is estimated that more than 300 million patients worldwide have asthma.(GINA 2019) It is postulated that with a projected continued increase in patients living in urban areas in 2025, there is likely to be a continued increase in asthma similar to the rise seen in previous decades. It is thought that we will likely have an additional 100 million more patients with asthma by 2025 than in 2004.(Masoli 2004)
In order for patients with asthma to achieve and sustain symptom control, adequate self-management, adherence to prescribed therapies, and adequate device technique are essential.(Bidad 2018) Although there have been advances in asthma therapies, asthma is still poorly controlled; studies demonstrate that current asthma control falls far short of national asthma management targets.(Colice 2012) Uncontrolled asthma, which is identified by poor symptom control, frequent severe exacerbations, serious exacerbations causing at least one hospitalization, and airflow limitation (forced expiratory volume [FEV1] <80% predicted), has been identified in approximately half of children (46%) and adults (55%) with asthma.(Liu 2010; Peters 2007)
Uncontrolled asthma is linked to frequent and intense flare ups of their disease. These flare ups, or exacerbations, impact both patient and society. The CDC estimates that up to 62% of adults in the US have uncontrolled disease.(Asthmastats CDC.gov)
Difficult-to-treat asthma is defined as uncontrolled asthma despite step 4 or 5 treatment: medium- or high-dose inhaled corticosteroid (ICS) with a second controller, usually a long-acting beta agonist (LABA), or maintenance oral steroid. Patients are also considered difficult to treat if step 4-5 therapy is required to maintain good symptom control and to reduce the risk of exacerbations.(GINA 2019)
Severe asthma represents a subset of difficult-to-treat patients. These are patients whose symptoms are uncontrolled despite adherence with maximal optimized therapy and treatment of contributing factors like esophageal reflux, allergic rhinitis, and smoking. This term “severe” can also be used when a patient’s control worsens when high dose treatment is decreased.(GINA 2019; Fajt 2017)
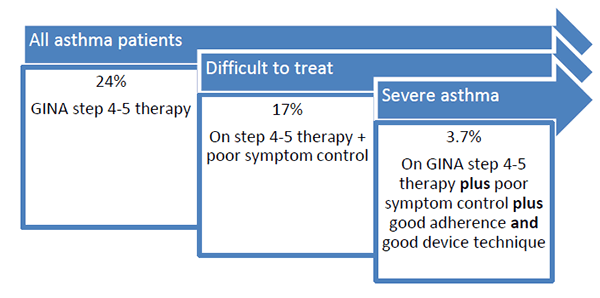
How common is severe asthma? As shown in Figure 1, approximately 24% of patients are on step 4-5 therapy; 17% of those have poor symptom control despite step 4-5 treatment, and <4% have severe asthma. Although this may not sound like a lot, patients with severe asthma represent approximately 50% of all costs associated with asthma.(ICER 2018)
Figure 1. Percentages of people with asthma and severity classifications per Global Initiative for Asthma (GINA).(GINA 2019)
With new advances and emerging treatment options, pharmacists are well positioned within the healthcare team to play an important role in the management of asthma, especially severe asthma, and can have a positive impact on asthma control, adherence to therapies, and patient outcomes.(Garcia-Cardenas 2016; Guénette 2015; Berry 2011; Bollmeier 2014)
SECTION 2: Advances in Understanding Severe Asthma
Clinical and mechanistic differences between mild and moderate asthma versus severe asthma
Most mild-moderate asthma cases are managed with inhalation therapies from four major pharmacological classes. Long term controllers include inhaled corticosteroids, long-acting bronchodilators, and leukotriene modifiers. Quick relief inhalers are typically short-acting beta agonists (SABA), but short-acting muscarinic antagonists (SAMA) can be used to decrease hospitalizations in the emergency department. Patients with persistent asthma are generally treated with ICS as they have the most evidence to support a reduction in airway remodeling and improved outcomes. However, their efficacy in patients with more severe disease is limited. This has led to the incorporation of poor response to ICSs (thereby necessitating the use of high doses of ICS) into recent definitions of severe asthma.(Fajt 2017) As previously mentioned, the subset of patients who remain difficult-to-treat represent a large portion of resource expenditure, and much remains unclear regarding the underlying causes of severe disease or best approaches to managing this disease.(Chung 2014)
If patients require step 4 or 5 therapy, due diligence is to ensure the diagnosis is correct and examine any contributing factors that could be leading to poor control including poor device technique and poor adherence. Treating co-morbid conditions like allergic rhinitis, gastroesophageal reflux disease (GERD), sleep apnea, and chronic sinusitis should also be done. Modifiable risk factors should be addressed. These include first- and second-hand smoke exposure, environmental and allergen exposure, and medications on board like non-steroidal anti-inflammatory drugs (NSAIDs) and beta blockers. Next, management should be optimized with ICS/LABA and the addition of tiotropium should be considered. Non-pharmacologic therapies include weight loss and ensuring proper vaccinations are up to date. Psychosocial problems like anxiety and depression should be addressed as these are common in patients with severe asthma and are associated with higher rates of exacerbations and emergency department visits.(Israel 2017)
Etiology and pathophysiology of severe asthma
The pathophysiology of asthma is complex, and many questions still exist regarding its etiology, especially concerning severe asthma. The pathogenesis of asthma involves the interaction of airway inflammation, variable airflow limitation, and bronchial hyperresponsiveness, mediated by immune response.(Colice 2012) We know the incidence and prevalence of asthma is much higher in developed Western countries compared with developing nations. This is thought to be due to our extremely clean household and work environments and fewer circulating infectious organisms. The “hygiene hypothesis” is based on the observation that the immune system is no longer adequately challenged in children born to parents in these industrialized nations due to a lack of exposure to infectious organisms.(Schroder 2008) In the hygiene hypothesis model, the developing immune system shifts the balance between what would normally be an equal number of T-helper (Th) cells type 1 and type 2.(Okada 2010) The lack of exposure to bacteria shifts the immune system from an “innate or non-allergic" immunity toward the Th2 cell mediated or “adaptive or allergic” type of immunity. This shift favors development of allergic disorders like allergic rhinitis, and asthma. Th2 cells produce interleukins: IL-4, IL-5, IL-6, and IL-13 which are all implicated in atopy through immune globulin (Ig) E, or IgE, production.
The traditional approach to asthma classification has been this assessment of atopic status and classification of ‘allergic’ versus ‘non-allergic’ asthma. Recently, it has been recognized that cellular inflammation and sequestration of eosinophils and neutrophils playing major roles. In this model, four distinct subtypes of asthma are proposed and are based on the presence or absence of eosinophils and/or neutrophils. These four subtypes include: eosinophilic asthma, neutrophilic asthma, granulocytic asthma (both eosinophils and neutrophils present), and paucigranulocytic asthma (eosinophils and neutrophils within normal levels).(Robinson 2017) Most patients with severe asthma display both eosinophilic and neutrophilic inflammation, but there is great variability in the numbers of each when sputum is analyzed, with patients demonstrating anywhere from none to very high levels of either cell. This number can also vary substantially from month to month.(Chung 2014) Neutrophilic asthma may be more prevalent in severe asthma, and it may be less responsive to corticosteroid treatment than eosinophilic asthma. It is also becoming apparent that asthma can also be categorized based on the degree of type 2 Th inflammation. Type 2 inflammation is now recognized as an important disease marker and a distinct endotype, while non-type 2-inflammatory versions of asthma may encompass several endotypes that are still under investigation (Figure 2).(Robinson 2017) Recent analyses hypothesize that several cytokine signaling pathways underlie the different types of severe asthma, but what these pathways are have not been fully described.(Poon 2016) It is important to note that “by identifying the distinct immunologic mechanisms involved in severe, poorly controlled asthma, new targeted therapies could improve patient quality of life and our understanding of human asthma.”(Fajt 2017)
Figure 2 below showcases the mechanisms of airway inflammation. Type 2 inflammation is most commonly initiated by the adaptive immune system when it recognizes allergens. These allergens act as antigens. Actions of the thymic stromal lymphopoietin (TSLP) stimulate Th2 cells and innate lymphoid cells of groups 2 (ILC2). These cells then differentiate and produce type 2 cytokines: IL-4, 5, and 13. These actions then lead to production of IgE and activation of mast cells as well as recruitment of eosinophils through IL-5. Smooth muscle is acted on by IL-13 which causes hypertrophy and remodeling. It also stimulates mucus production. Mast cells degranulate and release multiple inflammatory mediators that cause airway smooth muscle contraction, intensify the inflammatory cascade, and synthesize prostaglandin D2 (PGD2) which activates eosinophils through the receptor CRTH2.(Israel 2017)
The type 2 pathway can also be instigated by infectious organisms and irritants. These factors can stimulate the innate immune system through production of IL-33. These same initiating factors can also stimulate non-type 2 pathways. Type 17 helper (Th17) cells and its products stimulate neutrophils. This has led to therapeutic targets for severe asthma patients.(Israel 2017)
Figure 2. Pathways of airway inflammation in asthma.
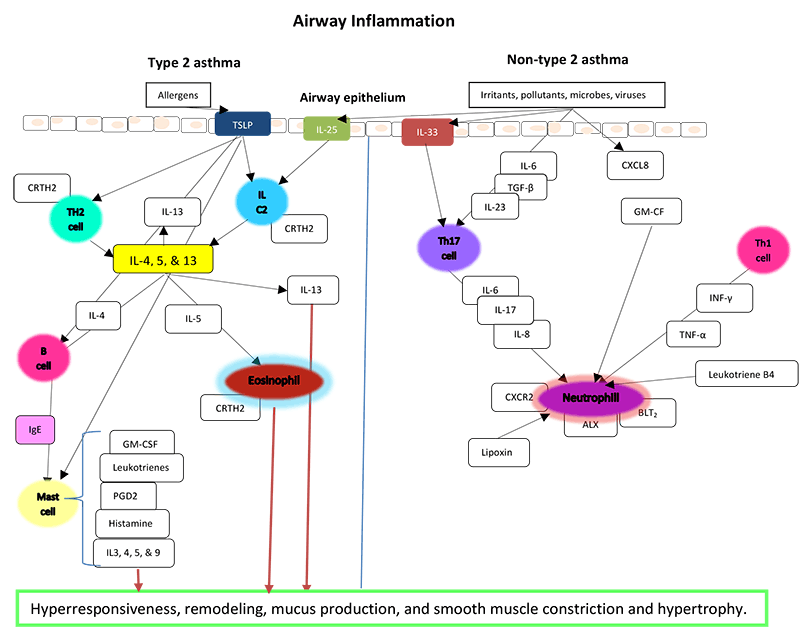
Adapted from: Israel E, Reddel HK. Severe and difficult to treat asthma in adults. New Engl J Med. 2017;377(10):965-76; Robinson D, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin & Experimental Allergy. 2017;(47):161-75; Diamant Z, et al. Biomarkers in asthma and allergic rhinitis. Pulmonary Pharmacology & Therapeutics. 2010;23:468-81.
| Table 1. Biomarkers and inflammation in severe asthma.(Israel 2017) |
| |
Type 2 asthma |
Non-type 2 asthma |
| Main cytokines |
IL-4, IL-5, IL-13 |
Possibly IL-17 |
| Cells involved |
Th2 cells
Eosinophils
Mast cells |
Th17 cells
Th1 cells
Neutrophils |
| Patient characteristics |
Elevated IgE
+ eosinophil count
(+) skin prick testing result |
No elevated IgE
(-) eosinophil count
(-) skin prick testing |
Because of the heterogenous nature of severe asthma, recent advances allow researchers to sub-define asthma based on clinical, pathophysiologic mechanisms, and cytokines or biomarkers (Table 1). Common ways severe asthma can be sub-defined include the use of phenotypes or endotypes. A phenotype refers to the observable characteristic of a specific disease entity. Phenotypes are clinically relevant because they relate to a patient presentation, asthma triggers, and treatment response.(Agache 2012) An endotype refers to the mechanisms of the underlying disease (Table 2). With the use of endotypes, researchers aim to separate characteristics by pathophysiological mechanism.(Wechsler 2018) To sub-define asthma by endotype, the use of biomarkers that relate to the underlying disease mechanism is required. Biomarkers come from body fluids and/or affected tissues.(Agache 2012)
| Table 2. Phenotypes and endotypes |
| Phenotype |
Endotype |
What can be observed clinically
Observable characteristic |
Pathophysiological mechanism
What is causing the disease? |
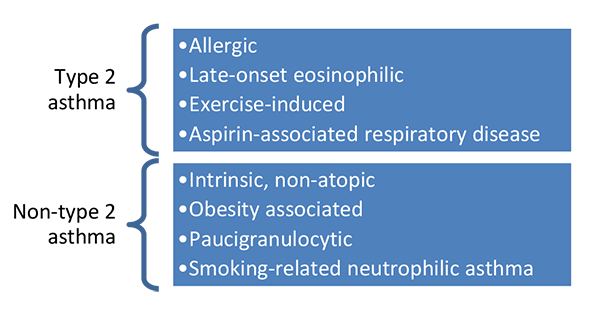
Traditionally, patients with asthma were classified based on observable characteristics or patterns like intrinsic or extrinsic asthma, or atopic and non-atopic. These however, don’t predict how well a patient will respond to therapies. Also, clinical observations of disease and disease severity and treatment response don’t always correlate well.(Dean 2017) This can lead to undesired adverse drug reactions as well as minimal effect of medications, and ultimately poor control of disease.(Dean 2017) In 2011, several pathophysiologically distinct asthma endotypes were defined. By sub-grouping patients into these endotypes, there is a potential to provide clinical separation and targeted treatment algorithms. The identified endotypes include allergic, exercise induced, as well as non-atopic, and obesity associated asthma (Figure 3).
Figure 3. Endotypes identified in asthma.(Dean 2017; Agache 2012; Fitzpatrick 2017)
There is further research ongoing in this area. The severe asthma research program (SARP) is a multicenter network in the US. Started in 2011 by the National Heart, Lung, and Blood Institute, it has been a forerunner in phenotype discovery in severe asthma patients.(Fitzpatrick 2018) Research continues to further identify both phenotypes and endotypes that will help guide future asthma therapies.
Identification of asthma sub-phenotypes has generally been through either clinical characteristics of subjects or through pathobiologic differences in sputum or bronchoscope specimens (Figure 4). The most-studied phenotypes are related to the age of the patient, age of asthma onset, and duration of asthma.(Wechsler 2018) All the biomarkers available in clinical practice are focused on type 2 or allergic inflammation. Examples include both sputum and blood eosinophils and exhaled nitric oxide (Table 3).(Fitzpatrick 2017)
Figure 4. Algorithm for assessing endotypes and phenotypes in severe asthma.
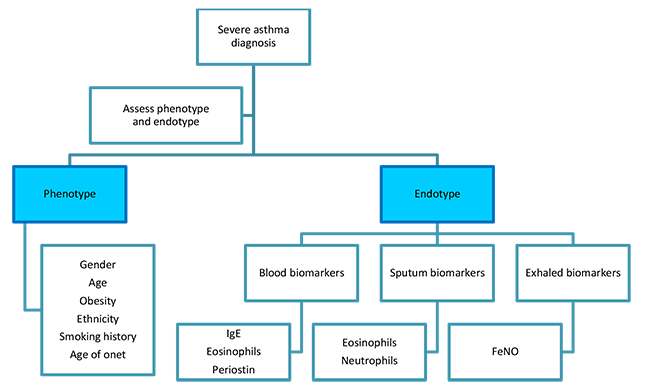
Adapted from Dunn RM, Wechsler ME. Anti-interleukin therapy in asthma. Clin Pharm Ther. 2015;97(1): 55-65.
| Table 3. Biomarkers utilized for patients with severe asthma.(Agache 2012; Izuhara 2016; Fitzpatrick 2017; Diamont 2010) |
| Test |
Where specimen is derived from in the body (how obtained) |
Cut-off value for asthma patients |
Clinical pearls |
| Eosinophils |
Blood
(blood draw) |
>150 cells/µL |
• Simple blood draw to obtain
• Affected by allergen exposure, steroids, and infection |
| Eosinophils |
Sputum
(induced sample) |
>3% |
• “Gold standard”
• Good correlation with Th2 asthma
• NOT widely available |
| FeNO (exhaled nitric oxide) |
Lungs
(breath test) |
>50 ppb |
• Simple, noninvasive test
• Can aid in asthma diagnosis
• Affected by ICS
• Should not be used as sole biomarker |
| Serum IgE |
Blood
(blood draw) |
>150 IU/ml |
• Identifies atopic phenotype |
| Serum periostin |
Blood
(blood draw) |
>50 ng/ml |
• Simple test
• Assumed to worsen airway inflammation
• Used with anti-IL-13 and anti-IgE to predict efficacy of these agents |
When contemplating initiating a biologic agent for patients with severe asthma, obtaining biomarkers of their disease is warranted. The testing results will help guide the therapy chosen. In general, a single biomarker may only capture a small fraction of the intervention effect, therefore it is important to sample multiple biomarkers if possible.(Diamant 2010) Overall, it is also thought to be best to obtain biomarkers from their relevant environment; for example, in the nose or lungs, as opposed to the serum or urine. Sputum eosinophils have long been considered the “gold standard” type 2 inflammatory biomarker as they are the best predictors of a clinical response to ICS therapy.(Diamant 2010) This however, can be problematic as it can be challenging for clinicians to find a lab that will analyze a sputum sample for inflammatory profiles.(Fitzpatrick 2017) Non-invasive tests like a blood eosinophil level and serum IgE are easily obtained, but it’s currently controversial whether these tests correlate well to airway eosinophilia.(Fitzpatrick 2017)
Exhaled nitric oxide (FeNO) is a sensitive marker of acute airway inflammation in allergic asthma which typically points to either uncontrolled disease or an exacerbation. A correlation has been shown between FeNO and an allergen induced late-asthmatic response. Also, several studies have shown a correlation between a loss of asthma control and an increase in this biomarker.(Diamant 2010) Periostin is a protein that activates inflammatory cells by binding to receptors and is deposited in the lung basement membrane in patients with asthma. Periostin is assumed to exacerbate airway allergic inflammation and has been used to predict efficacy of anti-IL-13 and anti-IgE agents.(Izuhara 2016) As previously stated, it is best to draw more than one biomarker as researchers think that patients with the greatest potential benefit from biologic therapies have elevations in more than one of these tests. These patients may eventually be further classified as “high responders.”(Fitzpatrick 2017)
CASE 1a
A 9-year old male patient, Johnny, has persistent asthma that is currently uncontrolled despite high dose ICS/LABA (fluticasone propionate/salmeterol 500/50 mcg 1 puff twice daily) and montelukast 5 mg once daily at bedtime.
Johnny was sent to an asthma specialist who wants to order tests to help assess his asthma phenotype. Johnny is terrified of getting any type of lab work done and the provider would like you to help put the patient and parents at ease.
CASE QUESTION
How would you best educate Johnny (and his parents) on the use of biomarkers to help assess his asthma phenotype?
PAUSE AND REFLECT: Assess case based on content thus far, then listen to and/or read the faculty commentary.
AUDIO CLIP: Case 1a question answered by the faculty.
|
SECTION 3: Evolving Therapies in Severe Asthma
Therapeutic targets in severe asthma
By better understanding the human immune system, researchers have been able to identify ways to interfere with disease and create medications with therapeutic targets. Several biologic therapies have been developed to target inflammatory pathways in patients with severe asthma (Figure 5).
Figure 5. Targets of biologic therapies in severe asthma.
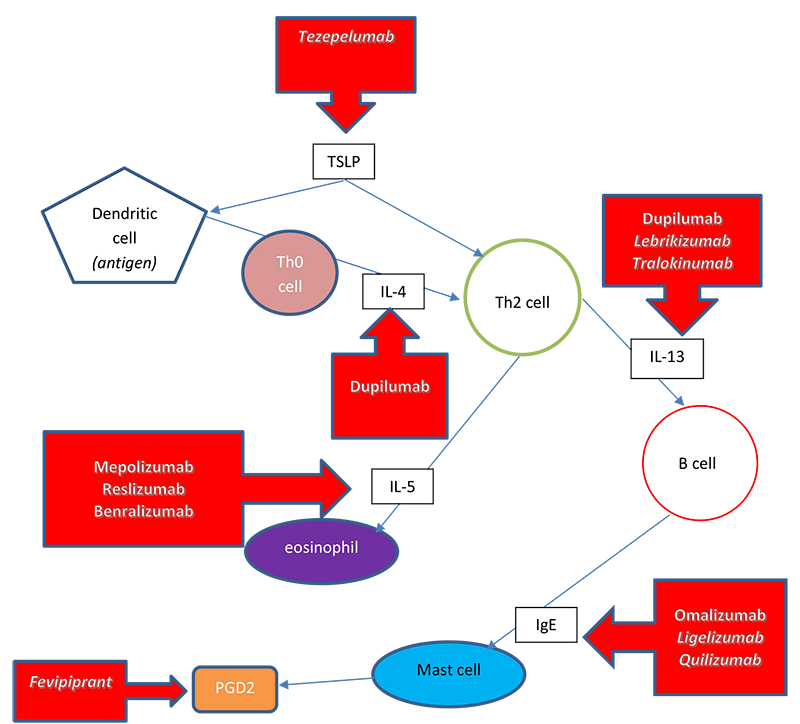
Adapted from: Israel E, Reddel HK. Severe and difficult to treat asthma in adults. New Engl J Med. 2017;377(10):965-76; Bice JB et al. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112:108-15.
Figure 5 illustrates where both currently available biologic therapies and investigational pipeline biologics play a role in helping to control airway inflammation in severe disease. The TSLP stimulates Th2 cells to differentiate and produce type 2 cytokines. Tezepelumab blocks TSLP, an epithelial cytokine, which may prevent the release of pro-inflammatory cytokines. This drug is in phase 3 studies and was given a breakthrough designation by the FDA in 2018. This designation was in response to data that showed a significant reduction in asthma exacerbations per year compared with placebo in patients with severe asthma. It is thought that since this drug blocks TSLP, multiple inflammatory pathways can be blocked “downstream.”(Press Release 2018)
Dupilumab is a fully human monoclonal antibody that is directed against the alpha subunit of the IL-4 receptor. This component signals both IL-4 and IL-13.(Rabe 2018) As previously discussed, IL-4 plays a key role in type 2 inflammation and induces airway remodeling in the lung.(Bice 2014) IL-13 is secreted primarily by Th2 cells and is involved in the regulation of IgE production, eosinophilic inflammation, airway smooth muscle contraction, and the recruitment of monocytes, macrophages, and T cells into the airway. IL-13 is a key target in the treatment of asthma and allergies. As mentioned earlier, periostin is a blood biomarker specific to IL-13. Periostin is surfacing as a good predictor of airway eosinophilia. Besides dupilumab, there are currently two drugs in development that bind to IL-13: lebrikizumab and tralokinumab.(Menzella 2017)
Interleukin-5 plays a major role in the activation and maturation of eosinophils at the site of inflammation. Due to this, we’ve seen the development of biologic therapies to specifically target IL-5 such as mepolizumab and reslizumab. Also, benralizumab targets the alpha chain of the IL-5 receptor.(Bice 2014)
The first biologic designed to treat patients with asthma that gained FDA approval was omalizumab, which binds to circulating IgE. In human studies, omalizumab has been shown to lower free IgE levels by 96-99%. Omalizumab also suppresses new IgE production and leads to a decrease in airway inflammation.(Bice 2014) Early studies show promise for ligelizumab and quilizumab; drugs in the pipeline that also target IgE.(Menzella 2017)
Fevipirant is an investigational agent that is an antagonist of the PGD2 receptor, CRTH2. This drug theoretically will bind to CRTH2 receptors on eosinophils, basophils, and T lymphocytes in the blood and tissues and inhibit their migration into airway tissue. This will then block the release of Th2 inflammatory cytokines.(Menzella 2017)
Rationale for use of specific interventions
As discussed, the role of biologic agents is still emerging. Each has unique characteristics, age indications, and dosing schemes. Some are available for self-injection; others must be given by intravenous (IV) infusion or in the provider’s office. Table 4 summarizes the FDA-approved biologics for use in patients with severe asthma; Table 5 shows drugs that are in development for asthma.
| Table 4. FDA-approved biologics for use in patients with severe asthma.(Package inserts; Chapman 2019) |
| Biologic agent |
Target |
FDA approved indication |
Age (yr) |
Dosing |
Clinical pearls |
| Omalizumab |
IgE |
Moderate/severe persistent asthma poor controlled despite ICS;
(+) skin prick testing to perennial aeroallergen (or in vitro reactivity) |
≥6 |
75-375 mg SQ q2-4 weeks |
• Dosing based on body weight and baseline serum IgE
• Reduces exacerbations by 40%; 60% if serum eosinophils >300 cells/µL
• Improves QOL
• Given by HCP only (SC)
• Allows for reduction in ICS dose and fewer oral corticosteroid bursts |
| Mepolizumab |
IL-5 |
Severe asthma and eosinophilic phenotype
To be added to maintenance therapy |
≥6 |
100 mcg SQ q4 weeks in adults and children ≥12 yr
40 mcg SQ q4 weeks in children ≥6-11 yr |
• May self-administer
• Reduces exacerbation rates by 47-53% overall and more if serum eosinophils are >500 cells/µL
• Improves FEV1 138-146 ml
• Improves QOL |
| Reslizumab |
IL-5 |
Severe asthma and eosinophilic phenotype
To be added to maintenance therapy |
≥18 |
3 mg/kg IV q4 weeks |
• Reduces exacerbation rates 50-60%
• Improves FEV1 100-160 ml
• IV infusion given in provider office |
| Benralizumab |
IL-5 rec |
Asthma poorly controlled on ICS/LABA and PO steroids; >2 exacerbations in past year; serum eosinophils
>300 cells/µl |
≥12 |
30 mg SQ q8 weeks |
• May be self-administered
• Reduces exacerbation rates by 55-70%
• Improves FEV1 256 ml
• Improves symptom scores
• Improves QOL
• Initial dosing: 30 mg SQ q 4 weeks x 3 doses |
| Dupilumab |
IL-4 and IL-13 |
Moderate to severe asthma with eosinophilic phenotype or with OCS dependent asthma
Also indicated in atopic dermatitis and chronic rhinosinusitis with nasal polyps |
≥12 |
200-300 mg SQ q other week |
• May be self-administered
• Initial dosing 400-600 mg SQ
• Reduces exacerbation rates by 59%
• If eosinophils ≥300 cells/µL; 71% lower exacerbation rates
• Reduces FeNO
• Improves spirometry
• Reduces oral steroid dosing |
| Abbreviations: yr: years; SQ: subcutaneous; QOL: quality of life; FeNO fractional exhaled nitric oxide; OCS: oral corticosteroid; PO: oral; ICS/LABA: inhaled corticosteroid, long-acting beta agonist; FEV1: forced expiratory volume in 1 second; HCP: healthcare provider; IgE: immune globulin E, IL: interleukin; rec: receptor |
| Table 5. Biologic agents in development for asthma.(Press Release 2018; Menzella 2017) |
| Drug |
Target |
| Tezepelumab |
TSLP |
Lebrikizumab
Tralokinumab |
IL-13 |
Ligelizumab
Quilizumab |
IgE |
| Fevipiprant |
PgD2 |
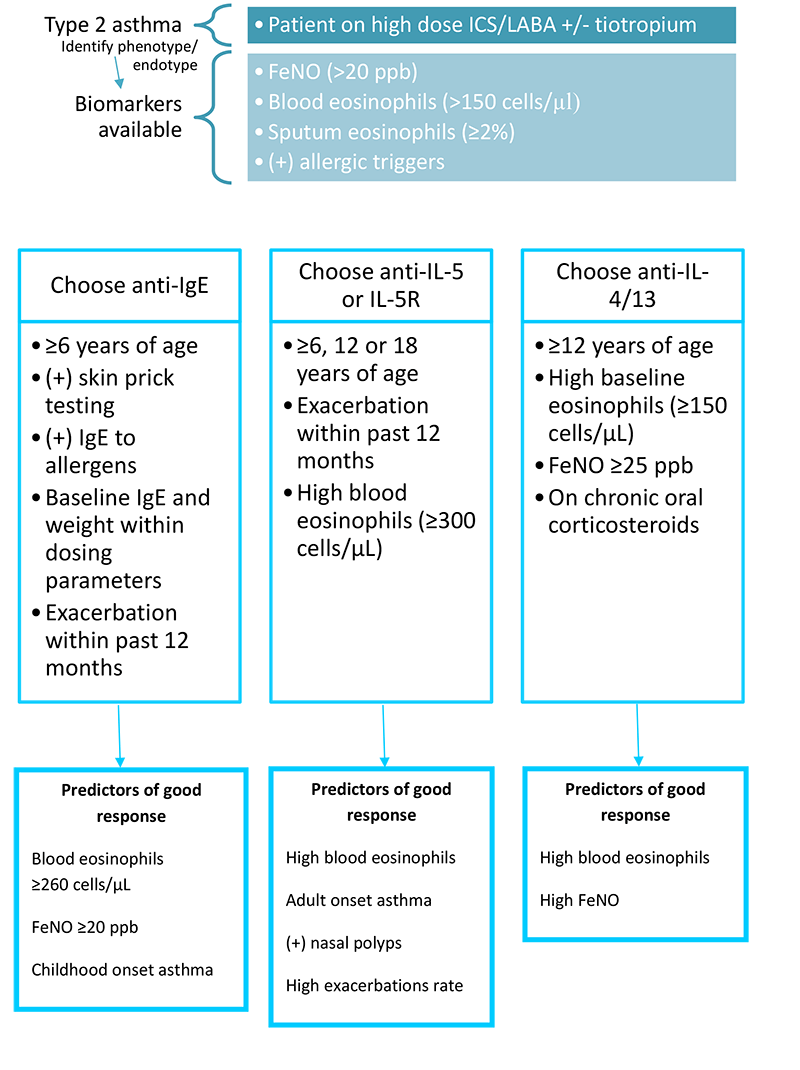
Which biologic agent to choose should be based on several factors including age of the patient, insurance coverage and cost, endotype, phenotype, and biomarker testing results. Other factors to consider include whether the patient desires to self-administer or travel to a provider’s office for injections. Refer to Figure 6 below regarding which biologic to choose for a given patient.
Figure 6. Selecting a biologic agent.(GINA 2019)
CASE 1b
Johnny, our patient from before, (9-year-old male; 50 kg) is to be initiated on a biologic for his asthma.
His biomarker results are as follows:
Blood IgE: 280 units/ml
Sputum eosinophils: Not obtained
Blood eosinophils: 290 cells/µl
Periostin: 45 ng/ml
FeNO: 30 ppb
Parents deny concomitant nasal polyps. Current medications include: fluticasone propionate/salmeterol 500/50 and montelukast.
CASE QUESTION
Which of the following is the most appropriate biologic to initiate for Johnny?
- Omalizumab
- Reslizumab
- Benralizumab
- Dupilumab
PAUSE AND REFLECT: Assess case based on content thus far, then listen to and/or read the faculty commentary.
AUDIO CLIP: Case 1b question answered by the faculty.
|
CASE 1c
How would your selection change if you patient was an adult? Harry, a 35-year-old male is to be initiated on a biologic for his asthma due to repeated exacerbations.
His biomarker results are as follows:
Blood IgE: not obtained
Sputum eosinophils: Not obtained
Blood eosinophils: 500 cells/µl
Periostin: 45 ng/ml
FeNO: 50 ppb
Harry’s medications include: fluticasone propionate/salmeterol 500/50 and montelukast 10 mg, and prednisone 10 mg every other day.
CASE QUESTION
Which of the following is the most appropriate biologic to initiate for Harry?
- Omalizumab
- Reslizumab
- Benralizumab
- Dupilumab
PAUSE AND REFLECT: Assess case based on content thus far, then listen to and/or read the faculty commentary.
AUDIO CLIP: Case 1c question answered by the faculty.
|
SECTION 4: Current Standards of Care for Severe Asthma
Global Initiative for Asthma (GINA)
The Global Initiative for Asthma (GINA) guidelines are updated annually (since 2002) and are created with primary care providers in mind. These recommendations are developed by experts from across the globe. Most US clinicians, however, are most familiar with the Expert Panel Report 3 (EPR-3) national asthma guidelines which are outdated. The GINA guidelines incorporate both newer treatment strategies as well as testing that is now available. Clinicians and patients benefit when incorporating these more updated recommendations into the treatment plans of their patients with asthma.
The GINA guidelines provide a step-wise approach for treating patients with asthma (including those with severe disease) and provides an easy-to-use figure (see GINA Pocket Guide link in Resource section); Table 6 summarizes the steps in that figure.
| Table 6. GINA step-wise approach to treating asthma. |
| |
|
|
|
Patients with severe asthma |
| |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
| Preferred controller |
As needed low dose budesonide + formoterol |
Daily low dose ICS
OR
As-needed low dose budesonide + formoterol |
Low dose ICS/LABA |
Medium dose ICS/LABA |
High dose ICS/LABA
Refer for phenotype assessment
+/- add on therapy (tiotropium, anti-IgE, anti-IL-5/5R, anti-IL-4R) |
| Other controller options |
Low dose ICS taken whenever SABA is taken |
Leukotriene receptor antagonist
OR
Low dose ICS taken whenever SABA taken |
Medium dose ICS
OR
Low dose ICS + LTRA |
High dose ICS,
Add–on tiotropium,
OR
add-on LTRA |
Add low dose oral corticosteroid, but consider ADRs |
| Preferred reliever |
As-needed low dose ICS/formoterol |
As-needed low dose ICS/formoterol for patients prescribed maintenance and reliever therapy |
| Other reliever option |
As-needed SABA |
| Adapted from GINA 2019 (page 46); Abbreviations: ICS/LABA: inhaled corticosteroids/long-acting beta agonist; SABA; short-acting beta agonist; LTRA: leukotriene receptor antagonists; ADRs: adverse drug reactions |
CASE 2
Sheila is a 22-year-old female with persistent asthma. She has questions for the pharmacist related to how long she’ll be on her mepolizumab, which was initiated 2 years ago. She says she doesn’t mind giving herself her monthly injection, but is worried that when she switches off her parent’s insurance next month, it won’t be covered by whatever insurance she’ll be able to purchase on the marketplace. She states she has seen a major reduction in exacerbation rates – from 3 per year to none since starting her therapy 2 years ago with mepolizumab – and was able to reduce her mometasone/formoterol from 200/5 1 puff two times per day to just mometasone 220 mcg 1 puff two times per day and has been able to stop her tiotropium Respimat altogether.
CASE QUESTION
How would you educate Sheila regarding the duration of her mepolizumab therapy?
PAUSE AND REFLECT: Assess case based on content thus far, then listen to and/or read the faculty commentary.
AUDIO CLIP: Case 2 question answered by the faculty.
|
SECTION 5: Pharmacist’s Role in Severe Asthma Management
Barriers to achieving optimal asthma control in patients with severe asthma
Obtaining and maintaining control of asthma is a priority and a goal of therapy.(GINA 2019) Unfortunately for many patients with asthma, achieving adequate control is difficult. Multiple studies have postulated reasons for the difficulty in achieving adequate asthma control. Factors identified include: wrong diagnosis, comorbid conditions like allergic rhinitis, incorrect treatment scheme, poor pulmonary device technique, inadequate trigger management (including current smoking), non-adherence to controller therapies, and individual variation in response to treatment.(Haughney 2008) As previously discussed, patients with severe disease may also have limited response to low-to-medium dose ICS therapies.
Pharmacist/provider
As mentioned, US guidelines (EPR-3) for treating asthma have not been updated in over ten years and do not include modern treatment approaches such as the use of tiotropium or any of the newer biologic agents available (mepolizumab, reslizumab, benralizumab, or dupilumab).(EPR3 2007) Omalizumab, the first biologic FDA-approved for asthma is mentioned, but the utility of this therapy has evolved since these guidelines were published. This has compelled providers to follow other recommendations such as the American Thoracic Society, European Respiratory Society, or the GINA strategies. Publishing guidelines, however, doesn’t always change prescribing or practices appreciably.(Guenette 2015)
The National Heart, Lung, and Blood Institute has convened an Expert Report Panel 4 working group. Draft updates to EPR-3 have been circulated for public comment and a revised version is anticipated in 2020.(EPR4 2020)
We know that pharmacists can improve outcomes in patients with asthma and it is recommended that pharmacists be part of multidisciplinary teams caring for patients with asthma.(Villamanan 2020)
Published studies show that pharmacist interventions have been associated with improvement in quality of life, more efficient medication use patterns, and improvement in disease control.(Villamanan 2020; Guenette 2015) Pharmacists are expertly trained in pharmacokinetics and pharmacodynamics of medications and are skilled in educating patients regarding pulmonary device techniques.(Villamanan 2020) Patient education and self-management strategies are key outcomes in patients with asthma. Educating patients related to their pulmonary device should be left to the medication expert. Community pharmacists are accessible members of the healthcare team and patients may visit them as often as every 30-90 days for refills.(Bollmeier 2014) Also, brief communications with a pharmacist can happen at no cost to the payer and without an appointment. Because pharmacists have access to medication use data and patients, pharmacists can readily identify medication use issues. Example issues include overuse of quick relief inhalers, lack of or non-adherence to controller agents, and monotherapy with a LABA.(Bollmeier, Prosser 2014) It is often thought that community pharmacists are a vast, largely untapped potential resource for patients needing help with their asthma control.(Berry 2011)
Patient
Poor asthma control affects the patient’s quality of life and has economic implications: these patients visit their providers more often for same day sick appointments, seek care at the emergency department frequently, and have lost productivity from work and school. We know that patients often misperceive their symptoms and often will rate their asthma as “fine” or “good.” In a recent survey of 1000 respondents with asthma (or parents), 78% reported their (or their child’s) overall health status as “good to excellent,” with only ~22% reporting “only fair,” “poor,” or “very poor.” Similarly, when patients not on controllers self-reported their level of asthma control, 64.5% felt their disease was either “completely” or “well-controlled.”(Colice 2012) In this same survey, 490 patients that were not on long-term controller therapy, but that were categorized as having more severe asthma, more frequently described feeling isolated or alone, fearful, depressed, and fatigued. This survey goes on to describe that most (79%) patients with asthma not on controller therapy did indeed have persistent disease, and most (85.7%) patients with asthma that were on controller therapy were not adequately controlling their disease.(Colice 2012)
Adherence to medications such as ICS is complex and can be affected by multiple factors. Non-adherence is thought to be caused by both unintentional (practical barriers) and intentional (related to motivation and beliefs) barriers. Cognitive and emotional illness as well as medication beliefs (personal convictions) directly influence a patient’s coping strategies and can affect intentional non-adherence.(Kosse 2019) Adherence rates in asthma patients are historically low (~50%) and have been reported to be even lower in adolescents. Some barriers specific to adolescents (12-18 years old) include forgetfulness and developmental norms. These patients have a desire to fit in with peer groups and they experience feelings of insecurity or invincibility. Also, this age group has unique medication beliefs; they feel that if they do not have symptoms, no medication is required.(Kosse 2019) Specific to adolescents, factors for non-adherence include: desire for independence and responsibility, parent-child conflicts, difficulties in setting priorities, lack of engagement in decision-making around medication use, not motivated, lack of perceived benefit, social stigma/embarrassment, and risk-taking behaviors including smoking tobacco or marijuana use.(Kaplan 2020) Other factors believed to influence adherence to asthma medications include: inadequate instruction, time consuming regimen, lack of communication with healthcare provider, prefer to treat immediate symptoms rather than prevent future ones, and impact of mental health (depression, anxiety).(Kaplan 2020)
Pharmacists should play a role in educating adult patients, adolescents, and parents stressing the importance of adherence, prioritizing their controller therapy, and making it part of their daily routine. Also, implementing technology reminders such as smart phone apps, or a simple alarm set on their phone, could help patients feel more engaged. Ensuring proper provider – patient communication and engaging adolescents in their choice of therapy, or preferred device, would also be helpful. Finally, helping patients understand trigger management including avoiding both first-and second-hand tobacco and marijuana smoke is key.
Another common reason for poor control of asthma is cost. The cost of treating asthma is immense and is derived from indirect, direct, and intangible costs. Total costs for treating patients with severe disease account for nearly triple the healthcare costs of those with persistent but non-severe asthma.(Chastek 2016) Treatment costs are derived from medications (e.g., drug costs and dispensing costs), laboratory testing, healthcare visits such as provider office as well as emergency department, and hospitalizations. A study conducted in Taiwan assessed a clinical pharmacist intervention on patients with moderate-to-severe asthma. Patients were educated about their disease and medications, given tools and education on self-management, and device and peak flow meter technique instruction was reviewed. Quality of life, patients’ asthma knowledge, and cost-effectiveness were also assessed. The authors found that after the intervention, patients’ quality of life, common knowledge about asthma, and peak expiratory flow rate (PEFR) were significantly improved compared to baseline. Frequency of use of quick relief medicines and oral steroids and total costs were reduced after the intervention period.(Chan 2004)
The pharmacist’s role in addressing barriers in severe asthma management
Pharmacists can and should play a key role in addressing barriers to care for patients with severe asthma. As mentioned previously, only appropriate eligible candidates should be given a trial of a biologic agent. Pharmacists can help ensure proper step therapy (high dose ICS/LABA +/- add-on with a leukotriene modifier or long-acting muscarinic antagonists [LAMA]) has been engaged prior to initiation of a biologic. Also, as medication experts, pharmacists are well-positioned within the healthcare team to assess controller adherence, adequate device technique, and ensure that trigger and environmental control measures have been employed. Next, pharmacists should recommend biomarker testing for patients when appropriate.
If a biologic agent is to be initiated, pharmacists should be helping patients navigate insurance coverage (medical vs. pharmacy benefit), prior authorization requirements, and educating the patient on whether the drug will be self-administered or done in the provider’s office. Also, the pharmacist should educate patients and caregivers about how these medications get to the patient (specialty pharmacist vs. home delivery) and if other support is needed, such as coupons or patient assistance programs. Similar to dispensing other medications, possible adverse effects should be reviewed and how to monitor for improvement in outcomes should be addressed. Lastly, patients should be given information related to storage requirements.
Table 7 summarizes pharmacist strategies to support patient self-management and improve asthma control.
| Table 7. Pharmacist strategies to support patients with severe asthma.(Bollmeier 2014; Highly 2019) |
| What pharmacists can do for patients with severe asthma |
| All pharmacists |
• Stay abreast of current asthma treatment guidelines
• Stay up-to-date on contemporary pharmacotherapy options for asthma
• Demonstrate proper device technique and utilize teach-back when educating patients on their device
• Assist with product selection based on insurance coverage, patient preference, and patient’s ability to utilize a pulmonary device |
| Hospital-based pharmacists |
• Provide hospital discharge counseling related to asthma medications including long term controllers, quick relief medications, self-management techniques and asthma action plans
• Assist team with selection of step 4-5 therapy when appropriate, including biomarker testing
• Educate patients on biologic therapy if chosen upon discharge
• Assist patients with acquisition cost of biologic (coupon, manufacturer patient assistance program, insurance prior authorization, etc.)
• Assist with biologic obtainment through specialty pharmacy when appropriate |
| Clinic-based pharmacists |
• Assist with diagnosis of severe airway disease ensuring patients are adequately given a trial of high dose ICS/LABA
• Ensure addition of other step 4-5 therapies have been tried: tiotropium, montelukast
• Educate patient on disease mechanisms and mechanism of action of both long-term controllers and quick relief agents
• Discuss role of adherence with long-term controllers as it relates to disease control
• Review proper device technique and reassess at each office visit
• Assist with smoking cessation and other trigger management
• Assist with choosing biomarker(s) to further assess endotype/phenotype
• Review results of biomarker screen and assist with biologic selection based on results
• Assist patients with biologic cost as appropriate (coupon, manufacturer patient assistance program, insurance prior authorization, etc.)
• Assist patient with acquisition from specialty pharmacy as appropriate
• Monitor progress at each follow-up appointment assessing symptom burden, quality of life, adverse drug reactions, and exacerbation history.
• Monitor biomarker response (eosinophil counts, FeNO, etc.) |
| Community pharmacists |
• Utilize dispensing data to help providers and patients by increasing use of ICS and decreasing use of oral steroids
• Review device technique with each dispense
• Utilizing dispensing data, screen for overuse of SABA agents and monotherapy with LABA agents
• Help recognize and treat exacerbations
• Review for ADRs of medications
• Assist patients with smoking cessation and other trigger management techniques
• Field questions from patients related to their biologic agent including storage requirements
• Communicate concerns related to asthma therapy to providers |
CASE 3
Melissa, an adult patient, calls the pharmacy asking for advice about how to inject her benralizumab which was recently delivered by her specialty pharmacy to her door. Melissa states it has been stored in the refrigerator since she got it and is ready to give herself the shot today.
CASE QUESTION
How would you educate Melissa on the benralizumab injection?
PAUSE AND REFLECT: Assess case based on content thus far, then listen to and/or read the faculty commentary.
AUDIO CLIP: Case 3 question answered by the faculty.
|
SECTION 6: Issues in Managed Care
Costs of treating severe asthma
Patients with severe asthma have repeated asthma symptoms, acute flare-ups, and are at high risk of potential adverse drug reactions due to high doses and the number of prescribed therapies required to control their disease. Their quality of life is impaired, with a negative influence on productivity at work or school and a higher risk of death.
Those with uncontrolled asthma have up to a 4.6 greater chance of hospitalization compared to those without asthma and up to a 1.8 higher chance of emergency department visits, they also have lower productivity, are more likely to be unemployed, spend more days absent from work, and have more activity limitations.(Nunes 2017) The cost of asthma increases as control decreases.(Nunes 2017) In a healthcare resource use study that included patients with asthma, all-cause healthcare costs averaged over $10,000 per patient with exacerbations, versus approximately $6,000 for those without exacerbations. Asthma-related healthcare costs were significantly higher among patients with severe persistent asthma experiencing exacerbations ($2663 vs. $1297).(Ivanova 2012)
Disease-related costs are classified into direct, indirect, and intangible costs. Direct costs are from managing the disease (visits to the emergency department, hospital stays, medications, outpatient visits, and testing). Indirect costs come from work-related losses and early mortality. Intangible costs stem from unquantifiable losses, for example, decreases in quality of life, increases in pain or suffering, and limitation of physical activities and job changes. On average, the annual cost to treat a patient with asthma is $5,000, but it is estimated to be much higher in patients with severe disease.(Nunes 2017) A 2009 review found the largest drivers of direct costs were medications, and of indirect costs were work and lost productivity at school.(Nunes 2017) Most studies show that direct costs account for between 50% and 80% of all total costs of asthma care. A retrospective review published in 2018 found the annual per-person incremental medical cost of asthma was $3,266 (2015 dollars) of which $1830 was from prescription medications. For indirect costs from 2008-2013, asthma was responsible for $3 billion in losses due to missed work and school days, and $29 billion in asthma-related mortality.(Nurmagambetov 2018) Older age, higher comorbidities, and male gender are other characteristics predictive of greater total asthma-related costs.(Chastek 2017)
With regard to indirect costs, when a patient is admitted to the hospital for an asthma exacerbation, the average number of working days lost is 13, with an average hospital length of stay of 4 days. Similarly, when a child misses school due to an exacerbation, on average they miss between 3-5 days of school. Presumably, the caregiver also loses this time at work.(Nunes 2017)
Asthma comorbidities also contribute to the cost of treating asthma. Examples of comorbid disease states include allergic rhinitis, GERD, obesity, and even depression and anxiety.
For patients with uncontrolled severe asthma, biologic therapy may be an option. The cost associated with this therapy is often a limitation as it can impact payers (including government-sponsored insurance programs), practitioners, and patients.(Anderson 2019) The average wholesale acquisition cost of one dose (individual unit) of biologics ranges from $879 to more than $4,000 (2018 dollars). These estimates do not include costs to practitioners who administer the medications, nor do they capture indirect patient costs associated with the time for administration.(Anderson 2019) A limitation when reviewing studies that try to quantify an economic benefit to biologic therapy is that drug prices are often based on wholesale price, which frequently will be different than actual pricing as payers typically negotiate a price.(Anderson 2019) While cost alone shouldn’t be the only driver in choosing a biologic agent, clinicians are often limited by insurance coverage, or a patient’s ability to pay which will drive decision making.
The Institute for Clinical and Economic Review (ICER) performed a review of the five FDA-approved biologic agents as add-on therapy for patients with uncontrolled moderate to severe asthma. The primary outcomes measures were reduction in asthma exacerbations and improvement in quality of life. Similar to previous findings, the authors found that all five drugs reduce annual exacerbation rates by ~50% and improvement in quality of life was seen. The authors also reported that the incremental cost-effective ratios for the five biologics were similar (ranging from $325,000 to $391,000 per quality adjusted life-year [QALY]). No biologic achieved a greater-than-zero likelihood of meeting the $150,000 for QALY or lower threshold. This informed the decision to deem all five biologics “low value.”(Tice 2019) It is estimated, that to be cost-effective, all biologics would have to reduce their cost by approximately 60% to 80%.(Anderson 2019)
Biologic agents are effective. Decreased monitoring costs of biologics and attenuation of risks of future exacerbations and hospitalizations with these agents do result in cost-effective care. This may justify the higher acquisition costs of these drugs.(Bukstein 2017) When considering the cost-effectiveness of biologic agents for patients with severe asthma, providers must consider all the costs and benefits. Value should be placed on long-term outcomes such as enhanced quality of life, cost of time lost from school or work, and reductions in the long-term economic productivity of the patient.(Bukstein 2017)
With respect to cost, when comparing the price of biologics to usual asthma pharmacotherapy, the break-even point of cumulative costs between treatment alternatives could be estimated. The break-even point is a point in time when cumulative costs of biologics equal cumulative costs of usual pharmacotherapy. Biologics tend to be more expensive in early years of therapy, but cumulative costs of pharmacotherapy start to exceed those associated with biologics (if other agents are able to be reduced or discontinued altogether). These break-even points in research are typically study specific and are not yet generalizable.(Bukstein 2017)
| What can payers do to help with the cost of biologics?(Tice 2019; Chapman 2019) |
• Negotiate preferential formulary status to biologic therapies with lower prices
• Develop prior authorization criteria that are reasonable ensuring only appropriate patients are given a biologic trial (consider step-up therapy before biologic is allowed)
• Continue to provide coverage of biologic agents for patients currently able to reduce ICS dose or other long-acting controller during treatment
• Work to ensure fair pricing is established and matched with low out-of-pocket costs for patients
• Limit prescribing of expensive biologics to specialists with expertise required to ensure appropriate and effective use of these agents (help provide effective referral pathway for primary care providers) |
Other issues related to managed care and severe asthma
There are no head-to-head randomized or observational trials of the five FDA-approved biologic agents for asthma. This makes it incredibly difficult to compare within the class. When a provider chooses a biologic, it would be beneficial to compare agents to each other, not just placebo.(Anderson 2019) The same holds true for insurers and payers. Also, there are important differences among them including indications such as age, severity of asthma, and differing phenotypes (Table 4).(Tice 2019) Similarly, when reviewing primary literature, there is much variation in methods utilized, outcomes measured, and prospective cost estimations.(McQueen 2018)
Determining appropriate duration of therapy and when to discontinue
There are currently no set strict recommendations regarding duration of biologic use. Initially, biologic agents should be given a trial of at least 4 months (GINA 2019) to determine if symptoms are improving or other objective evidence is present including a decrease in exacerbations. What does an adequate response to a biologic agent look like? Optimal response most likely resembles one that results in eliminating exacerbations, reduces oral steroid dosing (preferably to the point of discontinuation), and shows an improvement in quality of life.(Anderson 2019)
Most studies of biologics range from 15 months to 2 years in length. Long-term risks associated with the biologic agents will most likely have to continue to be determined through post marketing surveillance.(Anderson 2019) How long patients should remain on a biologic if responsive is an evolving question. One theory is that once control is achieved, patients should remain on their biologic for at least 3-6 more months. If stability persists, step-down therapy can be deployed. Discontinuation of any oral steroids should come first, then decrease or discontinue any add-on medications such as tiotropium, LABAs, and montelukast. Next, a reduction in ICS dose could be tried and finally, cessation of the biologic agent.(Highley 2019)
To improve cost-effectiveness, biologic treatment should be limited to responders. Biomarkers should be utilized to try to identify which patients are most likely to respond and biologics should be discontinued if no response is realized.(Anderson 2019)
Determining which biologic agent to place on a formulary
Improvements in our understanding of the pathophysiology of severe asthma, including the role of type 2 inflammation, have guided development of new biologic agents. Adding any of the five biologic agents available (omalizumab, benralizumab, reslizumab, mepolizumab, or dupilumab) to a plan’s formulary may provide significant benefit in appropriately selected patients. In general, all the FDA-approved biologics reduce the risk of asthma exacerbations and improve asthma-related quality of life in patients with severe IgE-mediated and/or eosinophilic phenotype. They also all improve FEV1.(Chapman 2019) Due to a lack of head-to-head comparisons, it is difficult to know which to consider “first line;” comparative research is needed. These drugs offer a viable treatment for patients with severe asthma when no other options exist. Their prices, however, are much higher than traditional therapies.
Payers inconsistently cover biologic agents. There appear to be differences in both initial access to a biologic and the duration authorized. For example, some plans have a lifetime cap on spending related to these agents regardless of efficacy. Some plans provide complete access, others may provide coverage for only 1-2 years, while others provider zero coverage.(Chapman 2019) Plans that cover physician-administered injectables (such as omalizumab) under their medical benefits are starting to move them to their pharmacy benefits. This makes it easier to subject them to utilization reviews. Also, plans that cover these under pharmacy benefits are increasingly requiring consumers to share the costs of these high-cost medications via coinsurance rather than co-payments. For example, a patient may be responsible for paying 25% coinsurance for high-cost drugs, with a maximum out of pocket expensive of $1,000 per year.(Goldman 2006)
Obviously, formulary restrictions help manage prescription drug spending. However, it is important that formulary restrictions also balance clinical, economic, and humanistic outcomes.(Happe 2014) Insurers should be creating ways to better utilize the biologics for severe asthma and helping patients that need them get access, rather than trying to deter their use due to cost.(Goldman 2006)
| What factors should be considered when selecting a biologic for a drug formulary? |
• Identify desired outcomes in patient population (i.e., reduction in oral corticosteroids, reduction in exacerbations, emergency department visits/hospitalizations, improvement in quality of life)
• Ability to track above outcomes
• FDA-approved indication of the biologic
• Safety profile of the biologic
• Updated asthma guidelines (GINA, and eventually EPR-4)
• Fair pricing is established matched with low out-of-pocket costs for patients
• Weigh price of biologic versus worsening asthma outcomes and break-even point
• Current drug utilization reviews |
SUMMARY SECTION
Treating patients with severe asthma is becoming less challenging as more targeted options become available. The use of biomarker testing and phenotypes to guide decision making is an important aspect of evolving treatment approaches, including when to consider biologic therapy. Biologic agents for severe asthma are extremely effective clinically, yet cost effectiveness remains less clear. Pharmacists are well positioned within the healthcare team to help a) educate patients; b) identify biomarkers needed; c) interpret testing results; d) select therapies for severe asthma; e) monitor response of therapy; and f) help patients acquire the best agent for their situation.
Resources
» Case Questions Transcript
REFERENCES
- Agache I et al. Untangling asthma phenotypes and endotypes. Allergy. 2012;67:835-46.
- Anderson WC, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma. To biologic or not to biologic? Ann All Asthma Immunol. 2019;122:367-72.
- Berry TM, Prosser TR, et al. Asthma Friendly Pharmacies: A model to improve communication and collaboration among pharmacists, patients, and healthcare providers. J Urban Health. 2011;88(1):s113-25.
- Bice JB et al. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112:108-15.
- Bidad N, Barnes N, et al. Understanding patients’ perceptions of asthma control: a qualitative study. Eur Respir J. 2018;51:1701346.
- Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks. Final evidence report. Midwest CEPAC. Available at: https://icer-review.org/wp-content/uploads/2018/04/ICER_Asthma_Draft_Report_092418v1.pdf Accessed March 10, 2020.
- Bollmeier SG, Prosser TR. Community pharmacy-based asthma services: current perspectives and future directions. Integrated Pharmacy Research and Practice. 2014;3:49-70.
- Bukstein DA, Luskin AT. Pharmacoeconomics of biologic therapy. Immunol Allergy Clin N Am. 2917;37:413-30.
- Centers for Disease Control and Prevention (CDC). Asthmastats. Available at https://www.cdc.gov/asthma/asthma_stats/. Accessed March 1, 2020.
- Centers for Disease Control and Prevention (CDC). National Asthma Data. Available at https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 1, 2020.
- Chan ALF, Wang HY. Pharmacoeconomic assessment of clinical pharmacist interventions for patients with moderate to severe asthma in outpatient clinics. Clin Drug Invest. 2004;24(10); 603-9.
- Chapman KR, Penz E, FitzGerald JM. Targeted management of severe asthma: Developing a Canadian approach. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2019. DOI: 10.1080/24745332.2019.1678443.
- Chastek B, Korrer S, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22(7):848-61.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS Guidelines on definition, evaluation, and treatment of severe asthma. Eur Respir J. 2014;43:343-73.
- Cinquair (reslizumab) [prescribing information]. Frazer, PA: Teva Pharmaceuticals; 2019.
- Colice GL, Ostrom NK, Geller DE, et al. The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States. Ann Allergy Asthma Immunol. 2012;108:157-62.
- Dean K, Niven R. Asthma phenotypes and endotypes: implications for personalized therapy. BioDrugs. 2017;31:393-408.
- Diamant Z, Boot JD, Mantzouranis E, et al. Biomarkers in asthma and allergic rhinitis. Pulmonary Pharmacology & Therapeutics. 2010;23:468-81.
- Dunn RM, Wechsler ME. Anti-interleukin therapy in asthma. Clin Pharm Ther. 2015;97(1):55-65.
- Dupixent (dupilumab) [prescribing information]. Tarrytown, NY: Regeneron; 2019.
- Fajt ML, Wenzel SE. Development of new therapies for severe asthma. All Asthma Immunol Res. 2017;9(1):3-14.
- Fasenra (benralizumab)[prescribing information]. Wilmington, DE: AstraZeneca; 2017.
- Fitzpatrick AM, Moore WC. Severe asthma phenotypes – how should they guide evaluation and treatment? I. 2017;5(4):901-8.
- Garcia-Cardenas V, Armour C, Benrimoj SI, et al. Pharmacists’ interventions on clinical asthma outcomes: a systematic review. Eur Respir J. 2016;47:1134-43.
- Global Initiative for Asthma (GINA). Available at ginasthma.org/reports. Accessed March 20, 2020.
- Goldman DP, Joyce GF, Lawless G, et al. Benefit design and specialty drug use. Health Affairs 2006;25(5):1319-31.
- Guénette L, Breton MC, Grégoire JP, et al. Effectiveness of an asthma integrated care program on asthma control and adherence to inhaled corticosteroids. J Asthma. 2015:52(6): 638-45.
- Happe LE, Clark D, Holliday E, et al. A systematic literature review assessing the directional impact of managed care formulary restrictions on medication adherence, clinical outcomes, economic outcomes, and health care resource utilization. JMCP. 2014;20(7): 677-84.
- Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102(12):1681-93.
- Highley AD, Cookman C, Morrow LE, et al. Severe asthma: An update for 2019. US Pharmacist. 2019;44(7):HS2-7.
- Press Release. Tezepelumab Granted Breakthrough Therapy Designation by US FDA. Available at https://www.astrazeneca.com/media-centre/press-releases/2018/tezepelumab-granted-breakthrough-therapy-designation-by-us-fda-07092018.html .
- Expert Panel Report 4 (EPR-4) Working Group. National Asthma Education and Prevention Program Coordinating Committee. Available at https://www.nhlbi.nih.gov/about/advisory-and-peer-review-committees/national-asthma-education-and-prevention-program-coordinating/EPR4-working-group.
- Israel E, Reddel HK. Severe and difficult to treat asthma in adults. New Eng J Med. 2017;377(10):965-76.
- Ivanova JI, Bergman R, Birnbaum HG, et al. Effect of asthma exacerbations on healthcare costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229-35.
- Izuhara K, Ohta S, Ono J. Using periostin as a biomarker in the treatment of asthma. All Asthma Immunol Res. 2016;8(6):491-8.
- Kaplan A, Price D. Treatment adherence in adolescents with asthma. J Asthma Allergy. 2010;13:39-49.
- Kosse RC, Koster ES, Kaptein AA, et al. Asthma control and quality of life in adolescents: the role of illness perceptions, medication beliefs, and adherence. J Asthma; DOI: 10.1080/02770903.2019.1635153.
- Liu AH, Gilsenan AW, Stanford RH, et al. Status of asthma control in pediatric primary care: results from the pediatric Asthma Control Characteristics and Prevalence Survey Study (ACCESS). J Pediatrics. 2010;157(2):276–81.
- Masoli M, Fabion D, Holt S, et al. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;39:469-78.
- McQueen RB, Sheehan DN, Whittington ND, et al. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. PharmacoEconomics. 2018;36:957-71.
- Menzella F, Galeone C, Bertolini F, et al. Innovative treatments for severe refractory asthma: how to choose the right option for the right patient? J Asthma Allergy. 2017;10:237-47.
- Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2020. Vital and Health Statistics 3. 2012;35:1-58.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
- Nucala (mepolizumab) [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2019.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1.
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Annals ATS. 2018;15(3):348-56.
- Okada H, Kuhn C, Feillet H, et al. The 'Hygiene Hypothesis' for autoimmune and allergic diseases: an update. Clin Exper Immunol. 2010;160:1-9.
- Peters SP, Jones CA, Haselkorn T, et al. Real-world evaluation of asthma control and treatment (REACT): Findings from a national web-based survey. J Allergy Clin Immunol. 2007;119(6):1454-61.
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. New Engl J Med. 2018;378:2475-85.
- Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin & Experimental Allergy. 2017;47:161-75.
- Schroder NWJ, Crother TR, Naiki Y, et al. Innate immune responses during respiratory tract infection with a bacterial pathogen induce allergic airway sensitization. J All Clin Immunol. 2008;122(3):595-602.
- Tice JA, Campbell JD, Synnot PG, et al. The effectiveness and value of biologic therapies for the treatment of uncontrolled asthma. A summary from the institute for clinical and economic review’s Midwest comparative effectiveness public advisory council. 2019;25(5): 510-13.
- Villamañán E, Herrero A, Álvarez-Sala R, Quirce S. Multidisciplinary severe asthma management: The role of hospital pharmacists in accredited specialized asthma units for adults in Spain [published online ahead of print, 2020 Jan 23]. J Investig Allergol Clin Immunol. 2020;0.
- Wechsler ME. Current and emerging biologic therapies for asthma and COPD. Respir Care. 2018;63(6):699-707.
- Xolair (omalizumab) [prescribing information]. South San Francisco, CA: Genentech; 2019.
Back to Top