
Expired activity
Please go to the PowerPak
homepage and select a course.
Straight to the Source: Top Questions Hospital Pharmacists Will Receive About Reversal of Factor Xa Inhibitors
Program Overview and Role of the Pharmacist
Direct factor Xa (FXa) inhibitors (rivaroxaban, apixaban, edoxaban, and betrixaban) have become the most widely utilized class of anticoagulants in the United States.1 Due to their expertise in managing anticoagulation and their role as the drug information experts, pharmacists are critically important in assuring the correct FXa inhibitor is being used in the correct indication, at the correct dose, with detailed patient education to maximize positive patient outcomes. They must also be able to recognize and manage bleeds that are associated with FXa inhibitors and provide guidance on when a reversal strategy may be necessary. This activity will provide pharmacists answers to the top questions they’ll be asked by fellow clinicians about reversal of FXa inhibitors and how to safely resume anticoagulation after a bleeding event.
Bleeding Risk of Currently Available FXa Inhibitors
A critical patient outcome for patients on FXa inhibitors is the monitoring and prevention of bleeding events. FXa inhibitors have demonstrated an improved safety profile compared to warfarin in randomized phase 3 trials. While the risk of intracranial hemorrhage (ICH) is reduced by approximately 50% with FXa inhibitors versus warfarin, they are not free of major bleeding events.2 The incidence of major bleeding with the use of an FXa inhibitor in patients receiving stroke prevention therapy for nonvalvular atrial fibrillation ranges from 2.1% to 3.6%, with rates of major gastrointestinal (GI) bleeding ranging from 0.8% to 3.2% and ICH ranging from 0.3% to 0.5%.3 In patients receiving an FXa inhibitor for the treatment of venous thromboembolism, the incidence of major bleeding ranges from 0.6% to 1.6%.4 Although some have suggested that one FXa inhibitor may be safer than another, no evidence from well-conducted trials supports this claim. These types of cross-trial comparisons are challenging, given different patient populations, as well as different definitions of major bleeding used in each clinical trial.3
Question 1: Does this patient need a reversal agent?
Although both major and nonmajor bleeds (criteria discussed below) will require some extent of supportive care, only life-threatening major bleeds or urgent surgery that cannot be delayed will require the use of a reversal agent. Supportive care includes discontinuation of other agents that may contribute to bleeding (antiplatelet agents and nonsteroidal anti-inflammatory drugs), direct compression or procedural management of the bleeding site, volume resuscitation with 0.9% NaCl or Ringer’s lactate, and blood product transfusion when appropriate.5 Patients with active bleeding should typically be transfused to maintain a hemoglobin of at least 7 g/dL, and patients with a history of coronary artery disease may benefit from a target of 8 g/dL.5
Pharmacists should be proficient at evaluating a number of nonmodifiable and modifiable clinical factors that place patients at higher risk of bleeding while receiving FXa inhibitors (Figure 1).6 By identifying these risk factors for bleeding, pharmacists can work with the healthcare team to modify a patient’s overall drug therapy in ways that reduce bleeding risk, such as changing the drug regimen to reduce the risk of hepatic or renal impairment, adjusting doses, or switching therapeutic agents.
| Figure 1. Factors that increase bleeding risk6 |
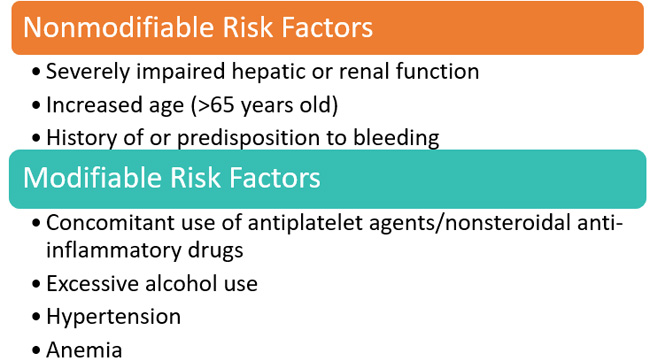 |
Bleeding events are typically classified as major or nonmajor. Major bleeding includes bleeding into a critical organ, bleeding leading to hemodynamic instability, bleeding producing a drop in hemoglobin of ≥2 units or the need for transfusion of ≥2 units of packed red blood cells (Figure 2).5
| Figure 2. Criteria for defining a major versus nonmajor bleed5 |
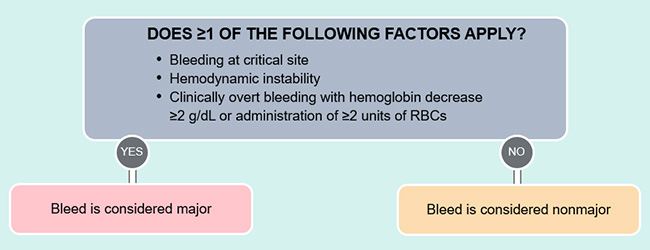 |
| RBCs, red blood cells. |
Bleeding into a critical organ includes ICH and other central nervous system bleeds and thoracic, intra-abdominal, retroperitoneal, intra-articular, and intramuscular bleeds. Intraluminal GI bleeding is not considered bleeding into a critical organ but may still qualify as major bleeding if there is hemodynamic instability or if hemoglobin and transfusion parameters are met. Major bleeding with hemodynamic instability typically presents with increased heart rate in an attempt to maintain perfusion with less volume. Systolic blood pressure <90 mmHg, a drop in systolic blood pressure >40 mmHg, or orthostatic blood pressure changes can represent hemodynamic instability. The best gauge of hemodynamic instability would be through intra-arterial measurement of mean arterial pressure, with a value of <65 mmHg being significant. Although a drop in hemoglobin ≥2 g/dL with clinically overt bleeding qualifies as a major bleed, many patients will not have a baseline hemoglobin from which to compare.5
Question 2: What labs should I order if the patient was on ____________?
Measurement of anticoagulant activity is a key step in the evaluation of an anticoagulated patient who presents with a clinically relevant bleed or needs an urgent unplanned procedure, but there are several complications when it comes to FXa inhibitors.5 More accessible tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) have limitations. The aPTT cannot be used for any meaningful evaluation of FXa inhibitors. Elevated PT can indicate therapeutic or supratherapeutic levels of FXa inhibitors, but a normal PT cannot be used for excluding increased risk of bleeding from apixaban.5,7 PT may provide a qualitative assessment for rivaroxaban and edoxaban, meaning that a normal PT could potentially exclude significant drug levels that could lead to increased bleeding risk, but this relies heavily on the sensitivity of the reagent used.8,9 International normalized ratio (INR) and dilute thrombin time cannot be used to measure anticoagulant activity of FXa inhibitors, and ecarin clotting time is unaffected by FXa inhibitors.5,10 FXa inhibitors can only be quantitatively measured by chromogenic anti-Xa assays that have to be calibrated for each specific agent, and there has not been widespread uptake of these assays. Recent studies have shown the potential to use the low molecular weight heparin (LMWH) calibrated anti-Xa assays for qualitative measurement of rivaroxaban and apixaban when the specific assay for these agents is not available.11,12
Laboratory measurements can be valuable in ambiguous clinical scenarios, such as when other factors may be contributing to the bleed (eg, a coagulopathy) or when the timing of the surgery needs to be determined. However, pharmacists do not have to confirm FXa inhibitor activity with a test before recommending for or against the use of a reversal agent. This decision should be made based on the specific clinical situation, the patient’s condition, and the severity of the bleed.
Question 3: The patient was on ____________. What should I use?
Currently, andexanet alfa is the only agent approved by the US Food and Drug Administration (FDA) for reversal of FXa inhibitors, specifically rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.13 Andexanet alfa is a modified recombinant protein derived from human coagulation FXa.14 Structural changes were made to maintain the active binding site to allow binding of FXa inhibitors while eliminating the enzymatic ability to convert prothrombin into thrombin. Therefore, andexanet functions as a decoy for binding FXa inhibitors and reverses their anticoagulant effect without producing pro- or anticoagulant effects itself. While andexanet can also bind to the tissue factor pathway inhibitor, an endogenous anticoagulant, this interaction is minimal in patients taking FXa inhibitors due to the higher binding affinity for FXa inhibitors.15 Guidance from the Anticoagulation Forum recommends andexanet first line and off-label 4F-PCC second line for the reversal of rivaroxaban and apixaban, and off-label andexanet or 4F-PCC for the reversal of betrixaban and edoxaban.16 Recommendations from the American College of Emergency Physicians, in press at the time of this activity's publication, are likely to be similar.17
The efficacy and safety of andexanet for reversal of acute major bleeding in patients who had received an FXa inhibitor within the previous 18 hours were evaluated in the ANNEXA-4 trial.18 The 2 co-primary outcomes of the trial were the change in anti-Xa activity and clinical hemostatic efficacy at 12 hours. Patients with low levels of anti-Xa activity at baseline (<75 ng/mL for FXa inhibitors and <0.25 IU/mL for enoxaparin) were included in the safety analysis but were not included in the efficacy analysis since they already had decreased anti-Xa activity. At the end of the trial, 254 patients were enrolled in the efficacy analysis and 352 patients in the safety analysis.
In patients receiving rivaroxaban (n=100) or apixaban (n=134), the anti-Xa activity was reduced by 92% at the end of the andexanet bolus, which was sustained to the end of the 2-hour infusion (Figure 3).18 Two hours after the infusion was completed (4 hours from baseline), anti-Xa activity increased but was still 42% and 32% lower than baseline, respectively. Hemostatic efficacy, as defined by the detailed criteria of the trial, was determined to be excellent or good in 82% of patients, and this finding was consistent regardless of the FXa inhibitor, gender, site of bleeding, age of the patient, or dose of andexanet given.
| Figure 3. Anti-Xa activity in the ANNEXA-4 efficacy population for apixaban and rivaroxaban18 |
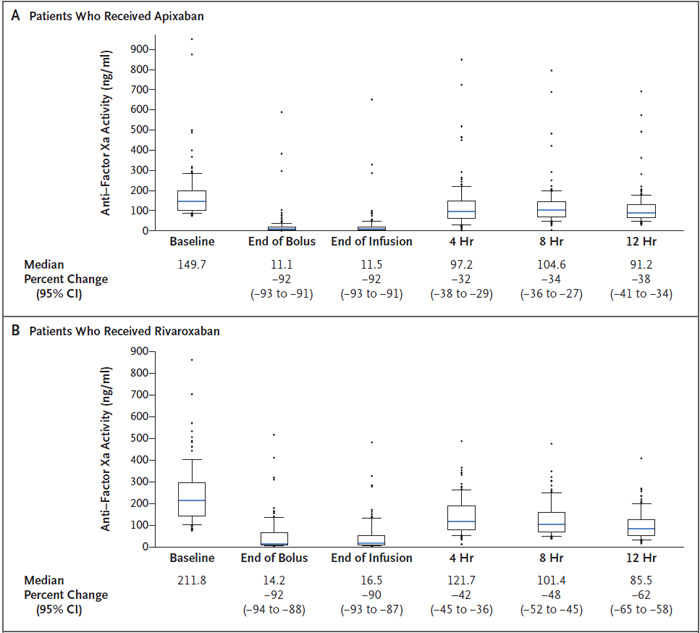 |
| |
Patients treated with andexanet in ANNEXA-4 had an overall thrombosis rate of 10% within 30 days and a 30-day mortality rate of 14%.18 Andexanet has a black box warning for thromboembolic risks, ischemic risks, cardiac arrest, and sudden death.13 Since these patients all had an indication for anticoagulant therapy, they were at risk of thrombotic events. It is suspected that reversal of the anticoagulant activity exposed this underlying pathophysiology. During the 30 days post-andexanet treatment, 62% of patients were restarted on oral or parenteral anticoagulation.18 The thrombosis rate in these patients after restarting any anticoagulation was only 2%, with zero events in patients after resuming oral anticoagulation. No antibodies to FX or FXa developed after andexanet treatment, and no neutralizing antibodies to andexanet developed.18 A recent substudy of ANNEXA-4 patients with traumatic ICH demonstrated good or excellent hemostatic efficacy in 84.5% of patients, and 30-day rates of thrombotic events and mortality were 7.1% and 10.1%, respectively.19 In a one-year, real-world, single-center study of patients with FXa-associated ICH, excellent or good hemostatic efficacy was observed in 82.6% of patients treated with andexanet, comparable to the ANNEXA-4 trial. No patients experienced a thrombotic event by hospital discharge or were readmitted within 30 days due to a thrombotic event.20
A nonspecific approach to reversal of FXa inhibitors has been to give prohemostatic agents, most commonly a 4-factor prothrombin complex concentrate (4F-PCC). This agent has factors II, VII, IX, and X and has 25-fold the concentration of clotting factors compared with plasma. Data with the use of PCCs are more limited. There have been trials in healthy volunteers demonstrating the ability to reverse measures of coagulation, but not anti-Xa levels.21-23 Both 4F-PCC and activated PCC (aPCC) are prothrombotic and have black box warnings for thromboembolic events.24,25
There have been 2 main prospective analyses of reversal of FXa-induced major bleeding with 4F-PCC.26,27 Schulman et al evaluated 66 patients and reported a rate of hemostatic efficacy of 85%.27 Majeed et al evaluated 84 patients and reported a rate of hemostatic efficacy of 69%.26 Although these appear comparable to the 82% demonstrated with andexanet alfa from the ANNEXA-4 trial, there are important differences in these trials. The hemostatic efficacy reported in these 4F-PCC trials was at 24 hours and not 12 hours as done in ANNEXA-4. The mean time from the last dose of FXa inhibitor was 18 hours in the Schulman et al study, compared to about 12 hours in ANNEXA-4, meaning that the amount of anticoagulant still in circulation was likely minimal.27 The time from last dose in the Majeed et al study was similar to ANNEXA-4, but the rate of hemostatic efficacy was then lower at the 24-hour evaluation time point.26 Finally, neither of these 4F-PCC trials evaluated for “on therapy” levels as was done in the ANNEXA-4 trial, in which patients with low levels of anti-factor Xa activity were excluded from the efficacy analysis. If the 28% rate of “below on therapy” levels remained consistent, these trials would only have evaluated approximately 47 or 48 and 60 or 61 patients, respectively.5,26,27 In these prospective trials, patients may have not had therapeutic levels of FXa inhibitors at the time of presentation and may have had similar outcomes with the cessation of anticoagulation alone.
Question 4: What dose should I use?
For andexanet alfa, clinicians should follow package insert recommended dosing, which considers time since the FXa inhibitor was last taken, along with drug and dose (Table 1).13 The low-dose regimen is a 400 mg bolus at 30 mg/minute followed by a 480 mg infusion over 2 hours. The high-dose regimen is an 800 mg bolus at 30 mg/min followed by a 960 mg infusion over 2 hours. The low-dose regimen utilizes 2 x 200 mg vials for the bolus and 3 x 200 mg vials for the infusion, while the high dose utilizes 4 x 200 mg vials for the bolus and 5 x 200 mg vials for the infusion.13 In the ANNEXA-4 study, approximately 84% of patients in the efficacy analysis received the low dose.18 Reconstitution of the 100 mg and 200 mg vials will be with 10 mL and 20 mL of sterile water for injection, respectively, and should occur by swirling gently, not shaking, to avoid foaming.13 Each vial takes 3 to 5 minutes to dissolve, so to minimize the total time needed for reconstitution, all vials needed for a patient’s treatment should be reconstituted in succession prior to dissolution.13 The cost of a low-dose regimen of andexanet is $24,750, with the high dose priced at $49,500, but a new technology add-on payment may reduce the cost by $14,000 in Medicare patients.28
| Table 1. Recommended andexanet alfa dosing13 |
| FXa Inhibitor |
Last Dose of
FXa Inhibitor |
Time From Last Dose |
| <8 Hours or Unknown |
≥8 Hours |
| Rivaroxaban |
≤10 mg |
Low Dose |
Low Dose |
| >10 mg or Unknown |
High Dose |
| Apixaban |
≤5 mg |
Low Dose |
| >5 mg or Unknown |
High Dose |
For nonspecific replacement strategies in patients taking direct oral anticoagulants (DOACs), studies with PCC/aPCC demonstrate widely varying doses from 10 to 50 units(U)/kg in vivo and >50 U/kg in animal models or in vitro.29,30 Only a few of these in vivo studies are small prospective cohorts, and many were case studies/series, retrospective, and/or low quality. The majority of data regarding these agents is limited (eg, in vitro studies) to coagulation factors being added to plasma from healthy volunteers taking DOACs and animal bleed models evaluating hemostatic efficacy.26,27 Dosing in recent studies of PCC/aPCC has trended down and been in the range of 8 to 35 U/kg, with 1 study suggesting a trend in improved mortality with doses <25 U/kg.26,27,29,31,32 Given the low quality of evidence and a recent trend in decreased dosing, 25 to 50 U/kg for life-threatening or critical site bleeds and lower dosing of 10 U/kg for other FXa inhibitor–related bleeds with options to re-dose, not to exceed the maximum of 5000 U, should be used.5,16,30 Fixed dosing of 1500 to 2000 U is an alternative dose strategy that has been recently studied and offers advantages of greater simplicity for the ordering provider and pharmacy and potentially reduced costs.16,27 Interestingly, 2 recent studies suggest that aPCC has no significant effect on anti-Xa levels until Xa inhibitor levels are almost negligible.33,34 The average cost of 4F-PCC using fixed dosing is approximately $2500 to $3500 with prices up to $8500 or higher, depending on the contract pricing, for weight-based, variable dosing.16
Question 5: Should I give another dose?
No repeat doses were given in the ANNEXA-4 trial.18 Thus, there is no current evidence to suggest that repeat dosing of andexanet alfa is beneficial, and there are currently no recommendations for repeat dosing. There may, however, be specific clinical scenarios such as trauma or overdose where providers and clinicians assess that benefit may outweigh risk for a repeat dose.
Repeated dosing of 4F-PCC is not well studied, and there is potential for prothrombotic complications.35,36 An additional dose of 4F-PCC has been used if cessation of bleeding was not achieved with a lower initial dose below the labeled maximum dose of 5000 U or if an INR could not be obtained at the time of administration of a lower dose.26,36
Question 6: When should I restart the anticoagulant?
While many of the life-threatening bleeds will initially be treated in the emergency department or in a critical care unit, patients with bleeding that is successfully treated need to have anticoagulation restarted as soon as safely possible.5 Reversal of the anticoagulant returns the patient to their elevated baseline thrombotic risk. Careful consideration and assessment of the benefits of restarting anticoagulation should be balanced with the risks of recurrent bleeding. These factors necessitate a personalized approach and discussion with each patient. In most cases, there is net clinical benefit to restarting anticoagulation after a bleeding event, especially in those at high thrombotic risk.37 It is important to note that certain patients may not benefit from restarting of anticoagulation due to their high of risk for recurrent bleeding. The American College of Cardiology suggests that paroxysmal atrial fibrillation with a CHA2DS2-VASc score ≤1 or a temporary indication for oral anticoagulation (eg, postsurgical prophylaxis, anticoagulation after an anterior myocardial infarction without left ventricle thrombus, recovered acute stress cardiomyopathy, first-time provoked venous thromboembolism >3 months ago, or bioprosthetic valve placement >3 months ago) would warrant discontinuing anticoagulation.5
The evidence for anticoagulation resumption after a bleed is derived mostly from observational studies. If anticoagulation needs to be restarted after GI bleeding, waiting approximately 14 days may be reasonable and provide the best balance between risk of GI bleed recurrence, thromboembolism, and mortality risk.38,39 Waiting 4 weeks after an ICH in patients without high thrombotic risk is the current recommendation.5,39,40 Step-up therapy is also a suggested approach, when feasible, to minimize the risk and impact of a recurrent bleed.5,41 This involves starting anticoagulants at a reduced dose and then increasing slowly to target dosing, while monitoring hemoglobin, hematocrit, platelets and signs and symptoms of bleeding.
Reversal Agents in Development
Ciraparantag (PER977) is a small synthetic, cationic, water-soluble molecule designed to bind to specific anionic molecules (ie, DOACs, unfractionated heparin, LMWH, fondaparinux) via noncovalent hydrogen bonding and charge-charge interactions.42 Ciraparantag binds to these anticoagulants by removing the drugs from their intended target and allowing rapid restoration of normal blood coagulation. Administered as a single dose, it has a rapid onset within 10 minutes and a sustained action of at least 24 hours. Its metabolite is primarily eliminated by renal excretion.42,43 Thus far, ciraparantag has shown no evidence of binding to a selected group of cardiovascular and antiepileptic drugs, but further evaluation of other medications will be needed to confirm the absence of off-target drug interactions.43
Ciraparantag has recently completed phase 1 studies in edoxaban and LMWH and initiated phase 2 trials for the reversal of apixaban and rivaroxaban.43-46 In phase 1 studies of healthy volunteers treated with a single dose of edoxaban or LMWH, ciraparantag demonstrated effective or complete reversal of anticoagulant activity with side effects including flushing, dysgeusia, and periorbital and facial warmth.43,44 Neither study showed procoagulant effects as measured by D-dimer, prothrombin fragment 1.2, and tissue factor pathway inhibitor at 12 and 24 hours after administration.43,44 Although promising, ciraparantag is in early development, and it remains to be seen how much of an impact it will have on the therapeutic landscape of reversal agents if approved by the FDA.
Conclusions
- You do not have to get laboratory confirmation of FXa inhibitor activity to use a reversal agent
- New guidelines and guidance statements highlight that andexanet alfa should be used first line for reversal of FXa inhibitors, specifically for rivaroxaban and apixaban (though some suggest off-label use for reversal of edoxaban and betrixaban), but PCCs can be used off-label for the reversal of FXa inhibitor activity if andexanet alfa is not available
- New data highlights that aPCC/PCC do not have any significant impact on anti-FXa levels in patients on FXa inhibitors, calling their use into question
- While most patients should resume their anticoagulation after a bleed at some point, consider the balance between thrombotic and hemorrhagic risk
References
- Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300-1305.
- Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34(7):489-498b.
- Dobesh PP, Fanikos J. Reducing The Risk Of Stroke In Patients With Nonvalvular Atrial Fibrillation With Direct Oral Anticoagulants. Is One Of These Not Like The Others? J Atr Fibrillation. 2016;9(2):1481.
- Dobesh PP, Fanikos J. New oral anticoagulants for the treatment of venous thromboembolism: understanding differences and similarities. Drugs. 2014;74(17):2015-2032.
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: A report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
- Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35(3):312-319.
- Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20(8):1231-1242.
- Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory Assessment of the Anticoagulant Activity of Direct Oral Anticoagulants: A Systematic Review. Chest. 2017;151(1):127-138.
- Konigsbrugge O, Quehenberger P, Belik S, et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol. 2015;94(9):1463-1471.
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467-1507.
- Billoir P, Barbay V, Joly LM, Fresel M, Chretien MH, Le Cam Duchez V. Anti-Xa Oral Anticoagulant Plasma Concentration Assay in Real Life: Rivaroxaban and Apixaban Quantification in Emergency With LMWH Calibrator. Ann Pharmacother. 2019;53(4):341-347.
- Yates SG, Smith S, Tharpe W, Shen YM, Sarode R. Can an anti-Xa assay for low-molecular-weight heparin be used to assess the presence of rivaroxaban? Transfus Apher Sci. 2016;55(2):212-215.
- Portola Pharmaceuticals, Inc. ANDEXXA Prescribing Information. https://www.fda.gov/media/113279/download. Published December 2018. Accessed September 5, 2019.
- Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446-451.
- Dobesh PP, Bhatt SH, Trujillo T, Glaubius K. Antidotes for reversal of direct oral anticoagulants. Pharmacol Therapeut. 2019: https://doi.org/10.1016/j.pharmthera.2019.107405. Accessed November 1, 2019.
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019;94(6):697-709.
- Baugh CW, Levine M, Cornutt D, et al. Anticoagulant Reversal Guidance Statement in the Emergency Department Setting: Recommendations of an Expert Panel. Ann Emerg Med. 2019, in press.
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335.
- Milling TJ, Yue P, Zotova E, et al. 199. Efficacy and Safety With Andexanet Alfa in Traumatic Intracranial Hemorrhage: An ANNEXA-4 Substudy. Ann Emerg Med. 2019;74(4):S78-S79.
- Concha M, Shomo E, Busey KV, Case D, Brockhurst A, Giovino A. 201. Outcomes Associated With Andexanet Use in Patients With Factor Xa Inhibitor-Associated Intracranial Hemorrhage: A One-Year Single-Center Analysis. Ann Emerg Med. 2019;74(4):S80.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
- Levi M, Moore KT, Castillejos CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost. 2014;12(9):1428-1436.
- Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation. 2015;131(1):82-90.
- CSL Behring. KCENTRA Prescribing Information. http://cslbehring.vo.llnwd.net/o33/u/central/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf. Published October 2018. Accessed November 1, 2019.
- Takeda. FEIBA Prescribing Information. https://www.shirecontent.com/PI/PDFs/FEIBA_USA_ENG.pdf. Published December 2018. Accessed November 1, 2019.
- Majeed A, Agren A, Holmstrom M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130(15):1706-1712.
- Schulman S, Gross PL, Ritchie B, et al. Prothrombin Complex Concentrate for Major Bleeding on Factor Xa Inhibitors: A Prospective Cohort Study. Thromb Haemost. 2018;118(5):842-851.
- Frontera J, Joset D, Lalchan R, Ahuja T, Papadopoulis J. Cost comparison of andexanet versus PCC for direct factor Xa inhibitor reversal after hemorrhage. Crit Care Med. 2019;47(1):416.
- Dager WE, Banares L. Reversing the anticoagulation effects of dabigatran. Hosp Pract (1995). 2017;45(2):29-38.
- Shaw JR, Siegal DM. Pharmacological reversal of the direct oral anticoagulants – A comprehensive review of the literature. Res Pract Thromb Haemost. 2018;2(2):251-265.
- Green L, Tan J, Antoniou S, et al. Haematological management of major bleeding associated with direct oral anticoagulants – UK experience. Br J Haematol. 2019;185(3):514-522.
- Dager WE, Roberts AJ, Nishijima DK. Effect of low and moderate dose FEIBA to reverse major bleeding in patients on direct oral anticoagulants. Thromb Res. 2019;173:71-76.
- Dzik WH. Reversal of oral factor Xa inhibitors by prothrombin complex concentrates: a re-appraisal. J Thromb Haemost. 2015;13:S187-S194.
- Lu G, Lin J, Bui K, Curnutte JT, Conley PB. PB1087. Contribution of Coagulation Factors in Prothrombin Complex Concentrates (PCCs) to TF-initiated Thrombin Generation in Normal and FXa Inhibitor-anticoagulated Plasma: The Relationship between Inhibitor Concentration and PCC-mediated Thrombin Generation. Res Pract Thromb Haemost. 2019;3(S1):802.
- Ghadimi K, Levy JH, Welsby IJ. Prothrombin Complex Concentrates for Bleeding in the Perioperative Setting. Anesth Analg. 2016;122(5):1287-1300.
- Unold D, Tormey CA. Clinical Applications of 4-Factor Prothrombin Complex Concentrate: A Practical Pathologist's Perspective. Arch Pathol Lab Med. 2015;139(12):1568-1575.
- Hernandez I, Zhang Y, Brooks MM, Chin PK, Saba S. Anticoagulation Use and Clinical Outcomes After Major Bleeding on Dabigatran or Warfarin in Atrial Fibrillation. Stroke. 2017;48(1):159-166.
- Chai-Adisaksopha C, Hillis C, Monreal M, Witt DM, Crowther M. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114(4):819-825.
- Witt DM. What to do after the bleed: resuming anticoagulation after major bleeding. ASH Education Book. 2016;2016(1):620-624.
- Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41(12):2860-2866.
- Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206-232.
- Hu TY, Vaidya VR, Asirvatham SJ. Reversing anticoagulant effects of novel oral anticoagulants: role of ciraparantag, andexanet alfa, and idarucizumab. Vasc Health Risk Manag. 2016;12:35-44.
- Ansell JE, Bakhru SH, Laulicht BE, et al. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117(2):238-245.
- Ansell JE, Laulicht BE, Bakhru SH, Hoffman M, Steiner SS, Costin JC. Ciraparantag safely and completely reverses the anticoagulant effects of low molecular weight heparin. Thromb Res. 2016;146:113-118.
- ClinicalTrials.gov. Phase 2 Study of Apixaban Reversal by Ciraparantag as Measured by WBCT. NCT03288454. Accessed September 6, 2019.
- ClinicalTrials.gov. Study of Ciraparantag Adminstered to Volunteers Anticoagulated with Rivaroxaban, Measure Clotting Times Using WBCT. NCT03172910. Accessed September 6, 2019.
Back to Top